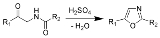
Robinson-Gabriel synthesis
Encyclopedia
The Robinson-Gabriel synthesis is a chemical reaction
that forms oxazole
s by dehydration
of 2-acylamino-ketone
s.
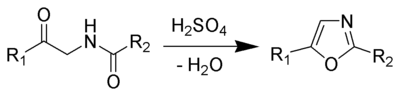 Historically, the dehydration agent is concentrated sulfuric acid
Historically, the dehydration agent is concentrated sulfuric acid
. Recently, phosphorus oxychloride is successful with this reaction also.
2-Acylamino-ketones can be synthesized using the Dakin-West reaction
.
Chemical reaction
A chemical reaction is a process that leads to the transformation of one set of chemical substances to another. Chemical reactions can be either spontaneous, requiring no input of energy, or non-spontaneous, typically following the input of some type of energy, such as heat, light or electricity...
that forms oxazole
Oxazole
Oxazole is the parent compound for a vast class of heterocyclic aromatic organic compounds. These are azoles with an oxygen and a nitrogen separated by one carbon. Oxazoles are aromatic compounds but less so than the thiazoles...
s by dehydration
Dehydration reaction
In chemistry and the biological sciences, a dehydration reaction is usually defined as a chemical reaction that involves the loss of water from the reacting molecule. Dehydration reactions are a subset of elimination reactions...
of 2-acylamino-ketone
Ketone
In organic chemistry, a ketone is an organic compound with the structure RCR', where R and R' can be a variety of atoms and groups of atoms. It features a carbonyl group bonded to two other carbon atoms. Many ketones are known and many are of great importance in industry and in biology...
s.

Sulfuric acid
Sulfuric acid is a strong mineral acid with the molecular formula . Its historical name is oil of vitriol. Pure sulfuric acid is a highly corrosive, colorless, viscous liquid. The salts of sulfuric acid are called sulfates...
. Recently, phosphorus oxychloride is successful with this reaction also.
2-Acylamino-ketones can be synthesized using the Dakin-West reaction
Dakin-West reaction
The Dakin–West reaction is a chemical reaction that transforms an amino-acid into a keto-amide using an acid anhydride and a base, typically pyridine. It is named for Henry Drysdale Dakin and Randolph West . Of special note, the keto-amide product is always racemic.With pyridine as a base and...
.

