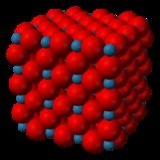
Rhenium trioxide
Encyclopedia
Oxide
An oxide is a chemical compound that contains at least one oxygen atom in its chemical formula. Metal oxides typically contain an anion of oxygen in the oxidation state of −2....
of the Group 7 elements (Mn
Manganese
Manganese is a chemical element, designated by the symbol Mn. It has the atomic number 25. It is found as a free element in nature , and in many minerals...
, Tc
Technetium
Technetium is the chemical element with atomic number 43 and symbol Tc. It is the lowest atomic number element without any stable isotopes; every form of it is radioactive. Nearly all technetium is produced synthetically and only minute amounts are found in nature...
, Re
Rhenium
Rhenium is a chemical element with the symbol Re and atomic number 75. It is a silvery-white, heavy, third-row transition metal in group 7 of the periodic table. With an average concentration of 1 part per billion , rhenium is one of the rarest elements in the Earth's crust. The free element has...
).
Structure
Rhenium oxide forms crystals with a primitivePrimitive cell
Used predominantly in geometry, solid state physics, and mineralogy, particularly in describing crystal structure, a primitive cell is a minimum cell corresponding to a single lattice point of a structure with translational symmetry in 2 dimensions, 3 dimensions, or other dimensions...
cubic
Cubic crystal system
In crystallography, the cubic crystal system is a crystal system where the unit cell is in the shape of a cube. This is one of the most common and simplest shapes found in crystals and minerals....
unit cell
Crystal structure
In mineralogy and crystallography, crystal structure is a unique arrangement of atoms or molecules in a crystalline liquid or solid. A crystal structure is composed of a pattern, a set of atoms arranged in a particular way, and a lattice exhibiting long-range order and symmetry...
, with a lattice parameter
Crystal structure
In mineralogy and crystallography, crystal structure is a unique arrangement of atoms or molecules in a crystalline liquid or solid. A crystal structure is composed of a pattern, a set of atoms arranged in a particular way, and a lattice exhibiting long-range order and symmetry...
of 3.742 Å
Ångström
The angstrom or ångström, is a unit of length equal to 1/10,000,000,000 of a meter . Its symbol is the Swedish letter Å....
(374.2 pm
Picometre
A picometre is a unit of length in the metric system, equal to one trillionth, i.e. of a metre, which is the current SI base unit of length...
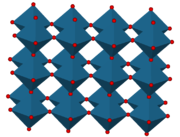
). The structure of ReO3 is similar to the Perovskite structure (ABO3), without the large A cation at the centre of the unit cell. Each rhenium atom is surrounded by six oxygen atoms, forming a ReO6 octahedron
Octahedron
In geometry, an octahedron is a polyhedron with eight faces. A regular octahedron is a Platonic solid composed of eight equilateral triangles, four of which meet at each vertex....
. These octahedra share corners to form the 3-dimensional structure.
Upon viewing a unit cell of ReO3, one can see that it is composed of eight ⅛ fragments of rhenium atoms, and twelve ¼ fragments of oxygen atoms. These numbers reduce to 1 Re and 3 O, hence the formula of ReO3. The coordination number of Re in this compound is 6 because each rhenium atom has six neighbouring oxygen atoms which bear opposite charge. The coordination number of O in this compound is 2 because each oxygen atom has 2 neighbouring Re atoms of opposite charge.
Properties
ReO3 is unusual for an oxide because it exhibits very low resistivityResistivity
Electrical resistivity is a measure of how strongly a material opposes the flow of electric current. A low resistivity indicates a material that readily allows the movement of electric charge. The SI unit of electrical resistivity is the ohm metre...
. It behaves more like a metal
Metal
A metal , is an element, compound, or alloy that is a good conductor of both electricity and heat. Metals are usually malleable and shiny, that is they reflect most of incident light...
in that its resistivity decreases as its temperature decreases. At 300 K
Kelvin
The kelvin is a unit of measurement for temperature. It is one of the seven base units in the International System of Units and is assigned the unit symbol K. The Kelvin scale is an absolute, thermodynamic temperature scale using as its null point absolute zero, the temperature at which all...
, its resistivity is 100.0 nΩ·m, whereas at 100 K, this decreases to 6.0 nΩ·m, 17 times less than at 300 K.
Preparation
Rhenium trioxide can be formed by reducing rhenium(VII) oxideRhenium(VII) oxide
Rhenium oxide is the chemical compound with the formula Re2O7. It is the anhydride of the hypothetical acid . Perrhenic acid, Re2O7·2H2O, however is closely related structurally to Re2O7. Solid Re2O7 is polymeric consisting, unusually, of the metal centers in two coordination environments...
with carbon monoxide
Carbon monoxide
Carbon monoxide , also called carbonous oxide, is a colorless, odorless, and tasteless gas that is slightly lighter than air. It is highly toxic to humans and animals in higher quantities, although it is also produced in normal animal metabolism in low quantities, and is thought to have some normal...
:
- Re2O7 + CO → 2 ReO3 + CO2

