
Response factor
Encyclopedia
Response factor, usually in chromatography
and spectroscopy
, is the ratio between a signal produced by an analyte, and the quantity of analyte which produces the signal. Ideally, and for easy computation, this ratio is unity (one). In real-world scenarios, this is often not the case.
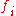 can be expressed on a molar, volume
can be expressed on a molar, volume
or mass
basis:
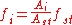
where A is the signal (e.g. peak area) and the subscript i indicates the sample and the subscript st indicates the standard
. The response factor of the standard is assigned an arbitrary factor, for example 1 or 100.
is unity, or very nearly so. Thus, by comparing the integrals of each peak observed in a proton NMR spectrum, one can determine composition of an arbitrary analyte mixture. Alternatively, one can determine the ratios of protons in various chemical environments in a single pure analyte.
On the other hand, for gas chromatography-mass spectrometry
, the total ion counts of different species in a mixture may not produce peaks of similar area per unit of analyte due to the different susceptibilities of fragmentation. As a result, comparing the peak areas of different compounds in a chromatogram, one may not determine the relative proportions of each compound in the mixture.
To compensate for this error, a known amount of an internal standard (a second compound that does not interfere with the analysis of the primary analyte) is added to all solutions (standards and unknowns). This way if the injection volumes (and hence the peak areas) differ slightly, the ratio of the areas of the analyte and the internal standard will remain constant from one run to the next.
This comparison of runs also applies to solutions with different concentrations of the analyte. The area of the internal standard becomes the value to which all other areas are referenced. Below is the mathematical derivation and application of this method.
Consider an analysis of octane (C8H18) using nonane (C9H20) as the internal standard. The 3 chromatograms below are for 3 different samples.
The amount of octane in each sample is different, but the amount of nonane is the same (in practice this is not a requirement). Due to scaling, the areas of the nonane peak appear to have different areas, but in reality the areas are identical. Therefore, the relative amounts of octane in each sample increases in the order of mixture 1 (least) < mixture 3 < mixture 2 (most).
This conclusion is reached because the ratio of the area of octane to that of nonane is the least in mixture 1 and the most, in mixture 2. Mixture 3 has an intermediate ratio. This ratio can be written as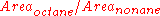 .
.
In chromatography, the area of a peak is proportional to the number of moles times some constant of proportionality, Area = k×n. The number of moles of compound is equal to the concentration (molarity, M) times the volume, n = MV. From these equations, the following derivation is made:
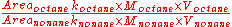
Since both compounds are in the same solution and are injected together, the volume terms are equal and cancel out. The above equation is then rearranged to solve for the ratio of the k's. This ratio is then called the response factor, F.
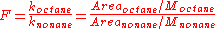
The response factor, F, is equal to the ratios of the k's, which are constant. Therefore F is constant. What this means is that regardless of the amounts of octane and nonane in solution, the ratio of the ratios of area to concentration will always yield a constant.
In practice, a solution containing known amounts of both octane and nonane is injected into a GC and a response factor, F, is calculated. Then a separate solution with an unknown amount of octane and a known amount of nonane is injected. The response factor is applied to the data from the second solution and the unknown concentration of the octane is found.
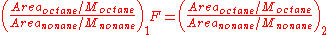
This example deals with the analysis of octane and nonane, but can be applied to any two compounds.
Chromatography
Chromatography is the collective term for a set of laboratory techniques for the separation of mixtures....
and spectroscopy
Spectroscopy
Spectroscopy is the study of the interaction between matter and radiated energy. Historically, spectroscopy originated through the study of visible light dispersed according to its wavelength, e.g., by a prism. Later the concept was expanded greatly to comprise any interaction with radiative...
, is the ratio between a signal produced by an analyte, and the quantity of analyte which produces the signal. Ideally, and for easy computation, this ratio is unity (one). In real-world scenarios, this is often not the case.
Expression
The response factor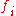 can be expressed on a molar, volume
can be expressed on a molar, volumeVolume
Volume is the quantity of three-dimensional space enclosed by some closed boundary, for example, the space that a substance or shape occupies or contains....
or mass
Mass
Mass can be defined as a quantitive measure of the resistance an object has to change in its velocity.In physics, mass commonly refers to any of the following three properties of matter, which have been shown experimentally to be equivalent:...
basis:
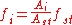
where A is the signal (e.g. peak area) and the subscript i indicates the sample and the subscript st indicates the standard
Standard (metrology)
In the science of measurement, a standard is an object, system, or experiment that bears a defined relationship to a unit of measurement of a physical quantity. Standards are the fundamental reference for a system of weights and measures, against which all other measuring devices are compared...
. The response factor of the standard is assigned an arbitrary factor, for example 1 or 100.
Examples
As a positive example, the response factors of each hydrogen atom in a sample analysed by proton NMRProton NMR
Proton NMR is the application of nuclear magnetic resonance in NMR spectroscopy with respect to hydrogen-1 nuclei within the molecules of a substance, in order to determine the structure of its molecules. In samples where natural hydrogen is used, practically all of the hydrogen consists of the...
is unity, or very nearly so. Thus, by comparing the integrals of each peak observed in a proton NMR spectrum, one can determine composition of an arbitrary analyte mixture. Alternatively, one can determine the ratios of protons in various chemical environments in a single pure analyte.
On the other hand, for gas chromatography-mass spectrometry
Gas chromatography-mass spectrometry
Gas chromatography–mass spectrometry is a method that combines the features of gas-liquid chromatography and mass spectrometry to identify different substances within a test sample. Applications of GC-MS include drug detection, fire investigation, environmental analysis, explosives investigation,...
, the total ion counts of different species in a mixture may not produce peaks of similar area per unit of analyte due to the different susceptibilities of fragmentation. As a result, comparing the peak areas of different compounds in a chromatogram, one may not determine the relative proportions of each compound in the mixture.
Chromatography
One of the main reasons to use response factors is to compensate for the irreproducibility of manual injections into a gas chromatograph (GC). Injection volumes for GC's can be 1 microliter (µL) or less and are difficult to reproduce. Differences in the volume of injected analyte leads to differences in the areas of the peaks in the chromatogram and any quantitative results are suspect.To compensate for this error, a known amount of an internal standard (a second compound that does not interfere with the analysis of the primary analyte) is added to all solutions (standards and unknowns). This way if the injection volumes (and hence the peak areas) differ slightly, the ratio of the areas of the analyte and the internal standard will remain constant from one run to the next.
This comparison of runs also applies to solutions with different concentrations of the analyte. The area of the internal standard becomes the value to which all other areas are referenced. Below is the mathematical derivation and application of this method.
Consider an analysis of octane (C8H18) using nonane (C9H20) as the internal standard. The 3 chromatograms below are for 3 different samples.
The amount of octane in each sample is different, but the amount of nonane is the same (in practice this is not a requirement). Due to scaling, the areas of the nonane peak appear to have different areas, but in reality the areas are identical. Therefore, the relative amounts of octane in each sample increases in the order of mixture 1 (least) < mixture 3 < mixture 2 (most).
This conclusion is reached because the ratio of the area of octane to that of nonane is the least in mixture 1 and the most, in mixture 2. Mixture 3 has an intermediate ratio. This ratio can be written as
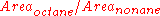 .
.In chromatography, the area of a peak is proportional to the number of moles times some constant of proportionality, Area = k×n. The number of moles of compound is equal to the concentration (molarity, M) times the volume, n = MV. From these equations, the following derivation is made:
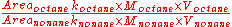
Since both compounds are in the same solution and are injected together, the volume terms are equal and cancel out. The above equation is then rearranged to solve for the ratio of the k's. This ratio is then called the response factor, F.
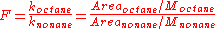
The response factor, F, is equal to the ratios of the k's, which are constant. Therefore F is constant. What this means is that regardless of the amounts of octane and nonane in solution, the ratio of the ratios of area to concentration will always yield a constant.
In practice, a solution containing known amounts of both octane and nonane is injected into a GC and a response factor, F, is calculated. Then a separate solution with an unknown amount of octane and a known amount of nonane is injected. The response factor is applied to the data from the second solution and the unknown concentration of the octane is found.
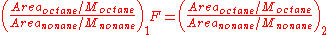
This example deals with the analysis of octane and nonane, but can be applied to any two compounds.

