
Replication timing
Encyclopedia
Replication Timing refers to the order in which segments of DNA along the length of a chromosome are duplicated.
(Figure 1), all of the DNA in a cell is duplicated in order to provide one copy to each of the daughter cells after the next cell division. The process of duplicating DNA is called DNA replication
, and it takes place by first unwinding the duplex DNA molecule, starting at many locations called DNA replication origins, followed by an unzipping process that unwinds the DNA as it is being copied. However, replication does not start at all the different origins at once. Rather, there is a defined temporal order in which these origins fire. Frequently a few adjacent origins open up to duplicate a segment of a chromosome, followed some time later by another group of origins opening up in an adjacent segment. Replication does not necessarily start at exactly the same origin sites every time, but the segments appear to replicate in the same temporal sequence regardless of exactly where within each segment replication starts. Figure 2 shows a cartoon of how this is generally envisioned to occur, while Figure 3 shows an animation of when different segments replicate in one type of human cell.
prior to cell division. The other way is to label newly synthesized DNA with chemically tagged nucleotides that become incorporated into the strands as they are synthesized, and then catch cells at different times during the duplication process and purify the DNA synthesized at each of these times using the chemical tag. In either case, we can measure the amount of the different DNA sequences along the length of the chromosome either directly using a machine that reads how much of each sequence is present or indirectly using a process called microarray hybridization. In any case, the temporal order of replication along the length of each chromosome can be plotted in graphical form to produce a "replication timing profile". Figure 4 shows an example of such a profile across 70,000,000 base pairs of human Chromosome 2 in a human embryonic stem cell line .
 At present, very little is known about either the mechanisms orchestrating the timing program or its biological significance. However, it is an intriguing cellular mechanism with links to many poorly understood features of the folding of chromosomes inside the cell nucleus. All eukaryotes have a timing program, and this program is similar in related species . This indicates that it is either important itself, or something important influences the program; in other words, it either represents or reflects something that Mother Nature wants to retain. It is unlikely that replicating DNA in a specific temporal order is necessary simply for the basic purpose of duplicating a DNA molecule. More than likely, it is related to some other chromosomal property or function. Replication timing is correlated with the expression of genes such that the genetic information being utilized in a cell is generally replicated earlier than the information that is not being used. We also know that the replication-timing program changes during development, along with changes in the expression of genes.
At present, very little is known about either the mechanisms orchestrating the timing program or its biological significance. However, it is an intriguing cellular mechanism with links to many poorly understood features of the folding of chromosomes inside the cell nucleus. All eukaryotes have a timing program, and this program is similar in related species . This indicates that it is either important itself, or something important influences the program; in other words, it either represents or reflects something that Mother Nature wants to retain. It is unlikely that replicating DNA in a specific temporal order is necessary simply for the basic purpose of duplicating a DNA molecule. More than likely, it is related to some other chromosomal property or function. Replication timing is correlated with the expression of genes such that the genetic information being utilized in a cell is generally replicated earlier than the information that is not being used. We also know that the replication-timing program changes during development, along with changes in the expression of genes.
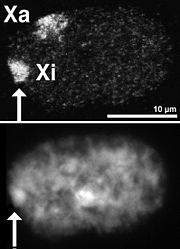
For many decades now, it has been known that replication timing is correlated with the structure of chromosomes. For example, female mammals have two X chromosomes. One of these is genetically active, while the other is inactivated early in development. In 1960, J. H. Taylor showed that the active and inactive X chromosomes replicate in a different pattern, with the active X replicating earlier than the inactive X, whereas all the other pairs of chromosomes replicate in the same temporal pattern. It was also noticed by Mary Lyon
that the inactive X took on a condensed structure in the nucleus called the Barr body
(Figure 5) at the same time during development as the genetic inactivation of the chromosome.
Altogether, this may not come as too much of a surprise, since the packaging of DNA with proteins and RNA into chromatin
takes place immediately after the DNA is synthesized. Therefore, replication timing dictates the time of assembly of chromatin. Less intuitive is the relationship between replication timing and the 3 dimensional positioning of chromatin in the nucleus. It is now well-accepted that chromatin is not randomly organized in the cell nucleus, but the positions of each chromosome domain relative to its neighboring domains
is characteristic of different cell types and after this geography is established in each newly formed cell, the chromosome domains do not move appreciably until the next cell division . Intriguingly, in all multi-cellular organisms where it has been measured, early replication takes place in the
interior of the nucleus and the chromatin around the periphery is replicated later. This compartmentalization seems to be quite profound, as recently developed methods to measure the points where different parts of chromosomes touch each other are almost perfectly aligned to when they replicate . In other words, regions that are replicated early vs. late are packaged in such a way as to be spatially segregated in the nucleus, with the intervening DNA containing the regions devoid of origin activity . One possibility is that these different
compartments within the nucleus, established and maintained without the aid of membranes or physical barriers, set thresholds for the initiation of replication so that the more accessible regions are the first to replicate .
DNA Replication
In eukaryotic cells (cells that package their DNA within a nucleus), chromosomes consist of very long linear double-stranded DNA molecules. During the S-phase of each cell cycleCell cycle
The cell cycle, or cell-division cycle, is the series of events that takes place in a cell leading to its division and duplication . In cells without a nucleus , the cell cycle occurs via a process termed binary fission...
(Figure 1), all of the DNA in a cell is duplicated in order to provide one copy to each of the daughter cells after the next cell division. The process of duplicating DNA is called DNA replication
DNA replication
DNA replication is a biological process that occurs in all living organisms and copies their DNA; it is the basis for biological inheritance. The process starts with one double-stranded DNA molecule and produces two identical copies of the molecule...
, and it takes place by first unwinding the duplex DNA molecule, starting at many locations called DNA replication origins, followed by an unzipping process that unwinds the DNA as it is being copied. However, replication does not start at all the different origins at once. Rather, there is a defined temporal order in which these origins fire. Frequently a few adjacent origins open up to duplicate a segment of a chromosome, followed some time later by another group of origins opening up in an adjacent segment. Replication does not necessarily start at exactly the same origin sites every time, but the segments appear to replicate in the same temporal sequence regardless of exactly where within each segment replication starts. Figure 2 shows a cartoon of how this is generally envisioned to occur, while Figure 3 shows an animation of when different segments replicate in one type of human cell.
Replication Timing Profiles
The temporal order of replication of all the segments in the genome, called its replication-timing program, can now be easily measured in two different ways . One way literally and simply measures the amount of the different DNA sequences along the length of the chromosome per cell. Sequences that duplicate first, long before cell division, will be more abundant in each cell than the sequences that replicate last justprior to cell division. The other way is to label newly synthesized DNA with chemically tagged nucleotides that become incorporated into the strands as they are synthesized, and then catch cells at different times during the duplication process and purify the DNA synthesized at each of these times using the chemical tag. In either case, we can measure the amount of the different DNA sequences along the length of the chromosome either directly using a machine that reads how much of each sequence is present or indirectly using a process called microarray hybridization. In any case, the temporal order of replication along the length of each chromosome can be plotted in graphical form to produce a "replication timing profile". Figure 4 shows an example of such a profile across 70,000,000 base pairs of human Chromosome 2 in a human embryonic stem cell line .
Replication Timing and Chromosome Structure

For many decades now, it has been known that replication timing is correlated with the structure of chromosomes. For example, female mammals have two X chromosomes. One of these is genetically active, while the other is inactivated early in development. In 1960, J. H. Taylor showed that the active and inactive X chromosomes replicate in a different pattern, with the active X replicating earlier than the inactive X, whereas all the other pairs of chromosomes replicate in the same temporal pattern. It was also noticed by Mary Lyon
Mary Lyon
Mary Mason Lyon , surname pronounced , was a pioneer in women's education. She established the Wheaton Female Seminary in Norton, Massachusetts, . Within two years, she raised $15,000 to build the Mount Holyoke School...
that the inactive X took on a condensed structure in the nucleus called the Barr body
Barr body
A Barr body is the inactive X chromosome in a female somatic cell, rendered inactive in a process called lyonization, in those species in which sex is determined by the presence of the Y or W chromosome rather than the diploidy of the X or Z...
(Figure 5) at the same time during development as the genetic inactivation of the chromosome.
Altogether, this may not come as too much of a surprise, since the packaging of DNA with proteins and RNA into chromatin
Chromatin
Chromatin is the combination of DNA and proteins that make up the contents of the nucleus of a cell. The primary functions of chromatin are; to package DNA into a smaller volume to fit in the cell, to strengthen the DNA to allow mitosis and meiosis and prevent DNA damage, and to control gene...
takes place immediately after the DNA is synthesized. Therefore, replication timing dictates the time of assembly of chromatin. Less intuitive is the relationship between replication timing and the 3 dimensional positioning of chromatin in the nucleus. It is now well-accepted that chromatin is not randomly organized in the cell nucleus, but the positions of each chromosome domain relative to its neighboring domains
is characteristic of different cell types and after this geography is established in each newly formed cell, the chromosome domains do not move appreciably until the next cell division . Intriguingly, in all multi-cellular organisms where it has been measured, early replication takes place in the
interior of the nucleus and the chromatin around the periphery is replicated later. This compartmentalization seems to be quite profound, as recently developed methods to measure the points where different parts of chromosomes touch each other are almost perfectly aligned to when they replicate . In other words, regions that are replicated early vs. late are packaged in such a way as to be spatially segregated in the nucleus, with the intervening DNA containing the regions devoid of origin activity . One possibility is that these different
compartments within the nucleus, established and maintained without the aid of membranes or physical barriers, set thresholds for the initiation of replication so that the more accessible regions are the first to replicate .

