
Regelation
Encyclopedia
Regelation is the phenomenon of melting under pressure and freezing again when the pressure is reduced. Many textbooks and reference books as well as websites often claim that regelation can be demonstrated by looping a fine wire around a block of ice , with a heavy weight attached to it. The pressure exerted on the ice slowly melts it locally, permitting the wire to pass through the entire block. The wire's track will refill as soon as pressure is relieved, so the ice block will remain solid even after wire passes completely through. This experiment is possible for ice at –10 °C or cooler, and while essentially valid, the details of the process by which the wire passes through the ice are complex . It has been suggested that the heating of the wire under pressure and tension may also play a role.
If 1 mm diameter wire is used, over an ice cube 50 mm wide, the area the force is exerted on is 50 mm2. This is 50 x 10-6 m2.
Force (in newtons) equals pressure (in pascals
) multiplied by area (in square metres).
If at least 500 atm (50 MPa) is required to melt the ice, a force of (50×106 Pa)(50×10-6 m2) = 2500 N is required, a force roughly equal to the weight of 250 kg
on Earth.
Regelation was discovered by Michael Faraday
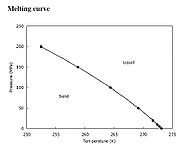
. Regelation occurs only for substances, such as ice, that have the property of expanding upon freezing, for the melting points of those substances decrease with increasing external pressure. The melting point
of ice
falls by 0.0072 °C for each additional atm of pressure applied. For example, a pressure of 500 atmospheres
is needed for ice to melt at –4 °C.
ice far below its melting point, there will be some relaxation of the atoms near the surface. Simulations of ice near to its melting point show that there is significant melting of the surface layers rather than a symmetric relaxation of atom positions. Nuclear magnetic resonance
provided evidence for a liquid layer on the surface of ice. In 1998, using atomic force microscopy
, Astrid Doppenschmidt and Hans Jurgen Butt, measured the thickness of the liquid-like layer on ice to be between 12 nm
at –24 °C and 70 nm at –0.7 °C. Surface melting was found to begin at temperatures as low as –33 °C.
The surface melting can account for the following:
If 1 mm diameter wire is used, over an ice cube 50 mm wide, the area the force is exerted on is 50 mm2. This is 50 x 10-6 m2.
Force (in newtons) equals pressure (in pascals
Pascal (unit)
The pascal is the SI derived unit of pressure, internal pressure, stress, Young's modulus and tensile strength, named after the French mathematician, physicist, inventor, writer, and philosopher Blaise Pascal. It is a measure of force per unit area, defined as one newton per square metre...
) multiplied by area (in square metres).
If at least 500 atm (50 MPa) is required to melt the ice, a force of (50×106 Pa)(50×10-6 m2) = 2500 N is required, a force roughly equal to the weight of 250 kg
Kilogram
The kilogram or kilogramme , also known as the kilo, is the base unit of mass in the International System of Units and is defined as being equal to the mass of the International Prototype Kilogram , which is almost exactly equal to the mass of one liter of water...
on Earth.
Regelation was discovered by Michael Faraday
Michael Faraday
Michael Faraday, FRS was an English chemist and physicist who contributed to the fields of electromagnetism and electrochemistry....
. Regelation occurs only for substances, such as ice, that have the property of expanding upon freezing, for the melting points of those substances decrease with increasing external pressure. The melting point
Melting point
The melting point of a solid is the temperature at which it changes state from solid to liquid. At the melting point the solid and liquid phase exist in equilibrium. The melting point of a substance depends on pressure and is usually specified at standard atmospheric pressure...
of ice
Ice
Ice is water frozen into the solid state. Usually ice is the phase known as ice Ih, which is the most abundant of the varying solid phases on the Earth's surface. It can appear transparent or opaque bluish-white color, depending on the presence of impurities or air inclusions...
falls by 0.0072 °C for each additional atm of pressure applied. For example, a pressure of 500 atmospheres
Atmosphere (unit)
The standard atmosphere is an international reference pressure defined as 101325 Pa and formerly used as unit of pressure. For practical purposes it has been replaced by the bar which is 105 Pa...
is needed for ice to melt at –4 °C.
Surface Melting
For a normal crystallineCrystal
A crystal or crystalline solid is a solid material whose constituent atoms, molecules, or ions are arranged in an orderly repeating pattern extending in all three spatial dimensions. The scientific study of crystals and crystal formation is known as crystallography...
ice far below its melting point, there will be some relaxation of the atoms near the surface. Simulations of ice near to its melting point show that there is significant melting of the surface layers rather than a symmetric relaxation of atom positions. Nuclear magnetic resonance
Nuclear magnetic resonance
Nuclear magnetic resonance is a physical phenomenon in which magnetic nuclei in a magnetic field absorb and re-emit electromagnetic radiation...
provided evidence for a liquid layer on the surface of ice. In 1998, using atomic force microscopy
Atomic force microscope
Atomic force microscopy or scanning force microscopy is a very high-resolution type of scanning probe microscopy, with demonstrated resolution on the order of fractions of a nanometer, more than 1000 times better than the optical diffraction limit...
, Astrid Doppenschmidt and Hans Jurgen Butt, measured the thickness of the liquid-like layer on ice to be between 12 nm
1 E-9 m
To help compare different orders of magnitudes this page lists lengths between 10−9 metres and 10−8 metres .Distances shorter than 1 nanometre*1 nm = 1 nanometre = 1000 picometres = 10 angstroms...
at –24 °C and 70 nm at –0.7 °C. Surface melting was found to begin at temperatures as low as –33 °C.

The surface melting can account for the following:
- Low coefficient of friction of ice, as experienced by skaters.
- Ease of compactionCompactionCompaction may refer to:* Soil compaction, for mechanically induced compaction near the ground surface* Compaction , part of the process of lithification involving mechanical dewatering of a sediment by progressive loading under several km of geomaterial* Waste compaction, related to garbage* Cold...
of ice - High adhesionAdhesionAdhesion is any attraction process between dissimilar molecular species that can potentially bring them in close contact. By contrast, cohesion takes place between similar molecules....
of ice surfaces
Examples of Regelation
- A glacierGlacierA glacier is a large persistent body of ice that forms where the accumulation of snow exceeds its ablation over many years, often centuries. At least 0.1 km² in area and 50 m thick, but often much larger, a glacier slowly deforms and flows due to stresses induced by its weight...
can exert a sufficient amount of pressure on its lower surface to lower the melting point of its ice. The melting of the ice at the glacier's base allows it to move from a higher elevation to a lower elevation. Liquid water may flow from the base of a glacier at lower elevations when the temperature of the air is above the freezing point of water.

