
Receptor-ligand kinetics
Encyclopedia
In biochemistry
, receptor-ligand kinetics is a branch of chemical kinetics
in which the kinetic species are defined by different non-covalent bindings and/or conformations of the molecules involved, which are denoted as receptor(s)
and ligand(s)
.
A main goal of receptor-ligand kinetics is to determine the concentrations of the various kinetic species (i.e., the states of the receptor and ligand) at all times, from a given set of initial concentrations and a given set of rate constants. In a few cases, an analytical solution of the rate equations may be determined, but this is relatively rare. However, most rate equations can be integrated numerically, or approximately, using the steady-state approximation
. A less ambitious goal is to determine the final equilibrium concentrations of the kinetic species, which is adequate for the interpretation of equilibrium binding data.
A converse goal of receptor-ligand kinetics is to estimate the rate constants and/or dissociation constant
s of the receptors and ligands from experimental kinetic or equilibrium data. The total concentrations of receptor and ligands are sometimes varied systematically to estimate these constants.
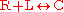
The equilibrium concentrations are related by the dissociation constant
Kd
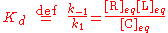
where k1 and k-1 are the forward and backward rate constants, respectively. The total concentrations of receptor and ligand in the system are constant
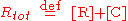
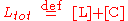
Thus, only one concentration of the three ([R], [L] and [C]) is independent; the other two concentrations may be determined from Rtot, Ltot and the independent concentration.
This system is one of the few systems whose kinetics can be determined analytically. Choosing [R] as the independent concentration and representing the concentrations by italic variables for brevity (e.g., ), the kinetic rate equation can be written
), the kinetic rate equation can be written
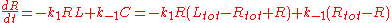
Dividing both sides by k1 and introducing the constant 2E = Rtot - Ltot - Kd, the rate equation becomes
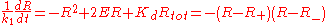
where the two equilibrium concentrations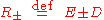 are given by the quadratic formula and the discriminant D is defined
are given by the quadratic formula and the discriminant D is defined
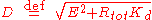
However, only the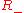 equilibrium is stable, corresponding to the equilibrium observed experimentally.
equilibrium is stable, corresponding to the equilibrium observed experimentally.
Separation of variables
and a partial-fraction expansion
yield the integrable ordinary differential equation
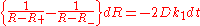
whose solution is
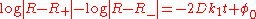
or, equivalently,
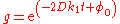
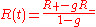
where the integration constant φ0 is defined
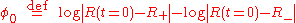
From this solution, the corresponding solutions for the other concentrations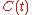 and
and 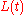 can be obtained.
can be obtained.
Biochemistry
Biochemistry, sometimes called biological chemistry, is the study of chemical processes in living organisms, including, but not limited to, living matter. Biochemistry governs all living organisms and living processes...
, receptor-ligand kinetics is a branch of chemical kinetics
Chemical kinetics
Chemical kinetics, also known as reaction kinetics, is the study of rates of chemical processes. Chemical kinetics includes investigations of how different experimental conditions can influence the speed of a chemical reaction and yield information about the reaction's mechanism and transition...
in which the kinetic species are defined by different non-covalent bindings and/or conformations of the molecules involved, which are denoted as receptor(s)
Receptor (biochemistry)
In biochemistry, a receptor is a molecule found on the surface of a cell, which receives specific chemical signals from neighbouring cells or the wider environment within an organism...
and ligand(s)
Ligand (biochemistry)
In biochemistry and pharmacology, a ligand is a substance that forms a complex with a biomolecule to serve a biological purpose. In a narrower sense, it is a signal triggering molecule, binding to a site on a target protein.The binding occurs by intermolecular forces, such as ionic bonds, hydrogen...
.
A main goal of receptor-ligand kinetics is to determine the concentrations of the various kinetic species (i.e., the states of the receptor and ligand) at all times, from a given set of initial concentrations and a given set of rate constants. In a few cases, an analytical solution of the rate equations may be determined, but this is relatively rare. However, most rate equations can be integrated numerically, or approximately, using the steady-state approximation
Steady state (chemistry)
In chemistry, a steady state is a situation in which all state variables are constant in spite of ongoing processes that strive to change them. For an entire system to be at steady state, i.e. for all state variables of a system to be constant, there must be a flow through the system...
. A less ambitious goal is to determine the final equilibrium concentrations of the kinetic species, which is adequate for the interpretation of equilibrium binding data.
A converse goal of receptor-ligand kinetics is to estimate the rate constants and/or dissociation constant
Dissociation constant
In chemistry, biochemistry, and pharmacology, a dissociation constant is a specific type of equilibrium constant that measures the propensity of a larger object to separate reversibly into smaller components, as when a complex falls apart into its component molecules, or when a salt splits up into...
s of the receptors and ligands from experimental kinetic or equilibrium data. The total concentrations of receptor and ligands are sometimes varied systematically to estimate these constants.
Kinetics of single receptor/single ligand/single complex binding
The simplest example of receptor-ligand kinetics is that of a single ligand L binding to a single receptor R to form a single complex C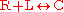
The equilibrium concentrations are related by the dissociation constant
Dissociation constant
In chemistry, biochemistry, and pharmacology, a dissociation constant is a specific type of equilibrium constant that measures the propensity of a larger object to separate reversibly into smaller components, as when a complex falls apart into its component molecules, or when a salt splits up into...
Kd
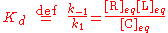
where k1 and k-1 are the forward and backward rate constants, respectively. The total concentrations of receptor and ligand in the system are constant
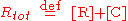
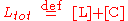
Thus, only one concentration of the three ([R], [L] and [C]) is independent; the other two concentrations may be determined from Rtot, Ltot and the independent concentration.
This system is one of the few systems whose kinetics can be determined analytically. Choosing [R] as the independent concentration and representing the concentrations by italic variables for brevity (e.g.,
 ), the kinetic rate equation can be written
), the kinetic rate equation can be written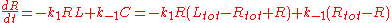
Dividing both sides by k1 and introducing the constant 2E = Rtot - Ltot - Kd, the rate equation becomes
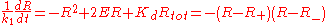
where the two equilibrium concentrations
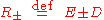 are given by the quadratic formula and the discriminant D is defined
are given by the quadratic formula and the discriminant D is defined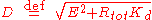
However, only the
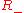 equilibrium is stable, corresponding to the equilibrium observed experimentally.
equilibrium is stable, corresponding to the equilibrium observed experimentally.Separation of variables
Separation of variables
In mathematics, separation of variables is any of several methods for solving ordinary and partial differential equations, in which algebra allows one to rewrite an equation so that each of two variables occurs on a different side of the equation....
and a partial-fraction expansion
Partial fraction
In algebra, the partial fraction decomposition or partial fraction expansion is a procedure used to reduce the degree of either the numerator or the denominator of a rational function ....
yield the integrable ordinary differential equation
Ordinary differential equation
In mathematics, an ordinary differential equation is a relation that contains functions of only one independent variable, and one or more of their derivatives with respect to that variable....
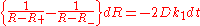
whose solution is
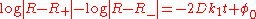
or, equivalently,
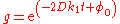
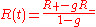
where the integration constant φ0 is defined
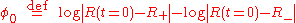
From this solution, the corresponding solutions for the other concentrations
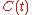 and
and 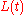 can be obtained.
can be obtained.Further reading
- D.A. Lauffenburger and J.J. Linderman (1993) Receptors: Models for Binding, Trafficking, and Signaling, Oxford University PressOxford University PressOxford University Press is the largest university press in the world. It is a department of the University of Oxford and is governed by a group of 15 academics appointed by the Vice-Chancellor known as the Delegates of the Press. They are headed by the Secretary to the Delegates, who serves as...
. ISBN 0-19-506466-6 (hardcover) and 0-19-510663-6 (paperback)

