
Protein kinase
Encyclopedia
Kinase
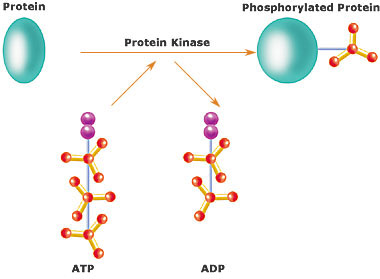
In chemistry and biochemistry, a kinase is a type of enzyme that transfers phosphate groups from high-energy donor molecules, such as ATP, to specific substrates, a process referred to as phosphorylation. Kinases are part of the larger family of phosphotransferases...
enzyme
Enzyme
Enzymes are proteins that catalyze chemical reactions. In enzymatic reactions, the molecules at the beginning of the process, called substrates, are converted into different molecules, called products. Almost all chemical reactions in a biological cell need enzymes in order to occur at rates...
that modifies other protein
Protein
Proteins are biochemical compounds consisting of one or more polypeptides typically folded into a globular or fibrous form, facilitating a biological function. A polypeptide is a single linear polymer chain of amino acids bonded together by peptide bonds between the carboxyl and amino groups of...
s by chemically adding phosphate
Phosphate
A phosphate, an inorganic chemical, is a salt of phosphoric acid. In organic chemistry, a phosphate, or organophosphate, is an ester of phosphoric acid. Organic phosphates are important in biochemistry and biogeochemistry or ecology. Inorganic phosphates are mined to obtain phosphorus for use in...
groups to them (phosphorylation
Phosphorylation
Phosphorylation is the addition of a phosphate group to a protein or other organic molecule. Phosphorylation activates or deactivates many protein enzymes....
). Phosphorylation usually results in a functional change of the target protein (substrate
Substrate (biochemistry)
In biochemistry, a substrate is a molecule upon which an enzyme acts. Enzymes catalyze chemical reactions involving the substrate. In the case of a single substrate, the substrate binds with the enzyme active site, and an enzyme-substrate complex is formed. The substrate is transformed into one or...
) by changing enzyme activity
Catalysis
Catalysis is the change in rate of a chemical reaction due to the participation of a substance called a catalyst. Unlike other reagents that participate in the chemical reaction, a catalyst is not consumed by the reaction itself. A catalyst may participate in multiple chemical transformations....
, cellular location, or association with other proteins. The human genome
Human genome
The human genome is the genome of Homo sapiens, which is stored on 23 chromosome pairs plus the small mitochondrial DNA. 22 of the 23 chromosomes are autosomal chromosome pairs, while the remaining pair is sex-determining...
contains about 500 protein kinase genes and they constitute about 2% of all human genes. Protein kinases are also found in bacteria and plants. Up to 30% of all human proteins may be modified by kinase activity, and kinases are known to regulate the majority of cellular pathways, especially those involved in signal transduction
Signal transduction
Signal transduction occurs when an extracellular signaling molecule activates a cell surface receptor. In turn, this receptor alters intracellular molecules creating a response...
.
Chemical activity
Amino acid
Amino acids are molecules containing an amine group, a carboxylic acid group and a side-chain that varies between different amino acids. The key elements of an amino acid are carbon, hydrogen, oxygen, and nitrogen...
s that have a free hydroxyl group. Most kinases act on both serine
Serine
Serine is an amino acid with the formula HO2CCHCH2OH. It is one of the proteinogenic amino acids. By virtue of the hydroxyl group, serine is classified as a polar amino acid.-Occurrence and biosynthesis:...
and threonine
Threonine
Threonine is an α-amino acid with the chemical formula HO2CCHCHCH3. Its codons are ACU, ACA, ACC, and ACG. This essential amino acid is classified as polar...
, others act on tyrosine
Tyrosine
Tyrosine or 4-hydroxyphenylalanine, is one of the 22 amino acids that are used by cells to synthesize proteins. Its codons are UAC and UAU. It is a non-essential amino acid with a polar side group...
, and a number (dual-specificity kinase
Dual-specificity kinase
In biochemistry, a dual-specificity kinase is a kinase that can act as both tyrosine kinase and serine/threonine kinase.MEKs, involved in MAP pathways, are principal examples of dual-specificity kinases...
s) act on all three. There are also protein kinases that phosphorylate other amino acids, including histidine kinase
Histidine kinase
Histidine Kinases are multifunctional, typically transmembrane, proteins of the transferase class that play a role in signal transduction across the cellular membrane. The vast majority of HKs are homodimers that exhibit autokinase, phosphotransfer, and phosphatase activity. HKs can act as...
s that phosphorylate histidine residues.
Regulation
Because protein kinases have profound effects on a cell, their activity is highly regulated. Kinases are turned on or off by phosphorylation (sometimes by the kinase itself - cis-phosphorylation/autophosphorylationAutophosphorylation
In biochemistry, autophosphorylation is the process in which a protein kinase attaches a phosphate group to itself. This usually lead to kinase activation or regulation, and phosphorylation of other kinase substrates....
), by binding of activator protein
Enzyme activator
Enzyme activators are molecules that bind to enzymes and increase their activity, and are often called coenzymes or cofactors. These molecules are often involved in the allosteric regulation of enzymes in the control of metabolism...
s or inhibitor protein
Enzyme inhibitor
An enzyme inhibitor is a molecule that binds to enzymes and decreases their activity. Since blocking an enzyme's activity can kill a pathogen or correct a metabolic imbalance, many drugs are enzyme inhibitors. They are also used as herbicides and pesticides...
s, or small molecules, or by controlling their location in the cell relative to their substrates.
Structure
The catalytic subunits of many protein kinases are highly conservedConserved sequence
In biology, conserved sequences are similar or identical sequences that occur within nucleic acid sequences , protein sequences, protein structures or polymeric carbohydrates across species or within different molecules produced by the same organism...
, and several structures have been solved.
Eukaryotic protein kinases are enzymes that belong to a very extensive family of proteins that share a conserved catalytic core. There are a number of conserved regions in the catalytic domain of protein kinases. In the N-terminal extremity of the catalytic domain there is a glycine
Glycine
Glycine is an organic compound with the formula NH2CH2COOH. Having a hydrogen substituent as its 'side chain', glycine is the smallest of the 20 amino acids commonly found in proteins. Its codons are GGU, GGC, GGA, GGG cf. the genetic code.Glycine is a colourless, sweet-tasting crystalline solid...
-rich stretch of residues in the vicinity of a lysine
Lysine
Lysine is an α-amino acid with the chemical formula HO2CCH4NH2. It is an essential amino acid, which means that the human body cannot synthesize it. Its codons are AAA and AAG....
amino acid, which has been shown to be involved in ATP binding. In the central part of the catalytic domain, there is a conserved aspartic acid
Aspartic acid
Aspartic acid is an α-amino acid with the chemical formula HOOCCHCH2COOH. The carboxylate anion, salt, or ester of aspartic acid is known as aspartate. The L-isomer of aspartate is one of the 20 proteinogenic amino acids, i.e., the building blocks of proteins...
, which is important for the catalytic activity of the enzyme.
Protein kinase groups
The human protein kinase family is divided into the following groups:- AGC kinases - containing PKA, PKCProtein kinase CProtein kinase C also known as PKC is a family of enzymes that are involved in controlling the function of other proteins through the phosphorylation of hydroxyl groups of serine and threonine amino acid residues on these proteins. PKC enzymes in turn are activated by signals such as increases in...
and PKG. - CaM kinases - containing the calcium/calmodulin-dependent protein kinases.
- CK1 - containing the casein kinase 1 group.
- CMGC - containing CDKCyclin-dependent kinasethumb|350px|Schematic of the cell cycle. outer ring: I=[[Interphase]], M=[[Mitosis]]; inner ring: M=Mitosis; G1=[[G1 phase|Gap phase 1]]; S=[[S phase|Synthesis]]; G2=[[G2 phase|Gap phase 2]]...
, MAPK, GSK3 and CLKCLK1Dual specificity protein kinase CLK1 is an enzyme that in humans is encoded by the CLK1 gene.-Further reading:...
kinases. - STE - containing the homologs of yeast Sterile 7, Sterile 11, and Sterile 20 kinases.
- TKTyrosine kinaseA tyrosine kinase is an enzyme that can transfer a phosphate group from ATP to a protein in a cell. It functions as an "on" or "off" switch in many cellular functions....
- containing the tyrosine kinases. - TKL - containing the tyrosine-kinase like group of kinases.
Serine/threonine-specific protein kinases

Serine
Serine is an amino acid with the formula HO2CCHCH2OH. It is one of the proteinogenic amino acids. By virtue of the hydroxyl group, serine is classified as a polar amino acid.-Occurrence and biosynthesis:...
or threonine
Threonine
Threonine is an α-amino acid with the chemical formula HO2CCHCHCH3. Its codons are ACU, ACA, ACC, and ACG. This essential amino acid is classified as polar...
(which have similar side-chains). Activity of these protein kinases can be regulated by specific events (e.g., DNA damage), as well as numerous chemical signals, including cAMP
Cyclic adenosine monophosphate
Cyclic adenosine monophosphate is a second messenger important in many biological processes...
/cGMP
Cyclic guanosine monophosphate
Cyclic guanosine monophosphate is a cyclic nucleotide derived from guanosine triphosphate . cGMP acts as a second messenger much like cyclic AMP...
, diacylglycerol
Diglyceride
A diglyceride, or a diacylglycerol , is a glyceride consisting of two fatty acid chains covalently bonded to a glycerol molecule through ester linkages....
, and Ca2+
Calcium in biology
Calcium plays a pivotal role in the physiology and biochemistry of organisms and the cell. It plays an important role in signal transduction pathways, where it acts as a second messenger, in neurotransmitter release from neurons, contraction of all muscle cell types, and fertilization...
/calmodulin
Calmodulin
Calmodulin is a calcium-binding protein expressed in all eukaryotic cells...
.
One very important group of protein kinases are the MAP kinases (acronym from: "mitogen-activated protein kinases"). Important subgroups are the kinases of the ERK subfamily, typically activated by mitogenic signals, and the stress-activated protein kinases JNK and p38.
While MAP kinases are serine/threonine-specific, they are activated by combined phosphorylation on serine/threonine and tyrosine residues. Activity of MAP kinases is restricted by a number of protein phosphatases, which remove the phosphate groups that are added to specific serine or threonine residues of the kinase and are required to maintain the kinase in an active conformation.
Two major factors influence activity of MAP kinases:
a) signals that activate transmembrane receptors (either natural ligands or crosslinking agents) and proteins associated with them (mutations that simulate active state)
b) signals that inactivate the phosphatases that restrict a given MAP kinase. Such signals include oxidant stress.
Tyrosine-specific protein kinases
TyrosineTyrosine
Tyrosine or 4-hydroxyphenylalanine, is one of the 22 amino acids that are used by cells to synthesize proteins. Its codons are UAC and UAU. It is a non-essential amino acid with a polar side group...
-specific protein kinases ( and ) phosphorylate tyrosine amino acid residues, and like serine/threonine-specific kinases are used in signal transduction
Signal transduction
Signal transduction occurs when an extracellular signaling molecule activates a cell surface receptor. In turn, this receptor alters intracellular molecules creating a response...
. They act primarily as growth factor
Growth factor
A growth factor is a naturally occurring substance capable of stimulating cellular growth, proliferation and cellular differentiation. Usually it is a protein or a steroid hormone. Growth factors are important for regulating a variety of cellular processes....
receptors and in downstream signaling from growth factors; some examples:
- Platelet-derived growth factor receptorPlatelet-derived growth factor receptorPlatelet-derived growth factor receptors are cell surface tyrosine kinase receptors for members of the platelet-derived growth factor family. PDGF subunits -A and -B are important factors regulating cell proliferation, cellular differentiation, cell growth, development and many diseases including...
(PDGFR) - Epidermal growth factor receptorEpidermal growth factor receptorThe epidermal growth factor receptor is the cell-surface receptor for members of the epidermal growth factor family of extracellular protein ligands...
(EGFR) - InsulinInsulinInsulin is a hormone central to regulating carbohydrate and fat metabolism in the body. Insulin causes cells in the liver, muscle, and fat tissue to take up glucose from the blood, storing it as glycogen in the liver and muscle....
receptorInsulin receptorIn molecular biology, the insulin receptor is a transmembrane receptor that is activated by insulin. It belongs to the large class of tyrosine kinase receptors....
and insulin-like growth factor 1 receptor (IGF1R) - Stem cell factor (SCF) receptor (also called c-kit, see the article on gastrointestinal stromal tumorGastrointestinal stromal tumorA gastrointestinal stromal tumor is one of the most common mesenchymal tumors of the gastrointestinal tract...
).
Receptor tyrosine kinases
These kinases consist of a transmembrane receptor with a tyrosine kinaseTyrosine kinase
A tyrosine kinase is an enzyme that can transfer a phosphate group from ATP to a protein in a cell. It functions as an "on" or "off" switch in many cellular functions....
domain protruding into the cytoplasm
Cytoplasm
The cytoplasm is a small gel-like substance residing between the cell membrane holding all the cell's internal sub-structures , except for the nucleus. All the contents of the cells of prokaryote organisms are contained within the cytoplasm...
. They play an important role in regulating cell division
Cell division
Cell division is the process by which a parent cell divides into two or more daughter cells . Cell division is usually a small segment of a larger cell cycle. This type of cell division in eukaryotes is known as mitosis, and leaves the daughter cell capable of dividing again. The corresponding sort...
, cellular differentiation
Cellular differentiation
In developmental biology, cellular differentiation is the process by which a less specialized cell becomes a more specialized cell type. Differentiation occurs numerous times during the development of a multicellular organism as the organism changes from a simple zygote to a complex system of...
, and morphogenesis
Morphogenesis
Morphogenesis , is the biological process that causes an organism to develop its shape...
. More than 50 receptor tyrosine kinases are known in mammals.
Structure
The extracellular domain serves as the ligandLigand (biochemistry)
In biochemistry and pharmacology, a ligand is a substance that forms a complex with a biomolecule to serve a biological purpose. In a narrower sense, it is a signal triggering molecule, binding to a site on a target protein.The binding occurs by intermolecular forces, such as ionic bonds, hydrogen...
-binding part of the molecule. It can be a separate unit that is attached to the rest of the receptor by a disulfide bond
Disulfide bond
In chemistry, a disulfide bond is a covalent bond, usually derived by the coupling of two thiol groups. The linkage is also called an SS-bond or disulfide bridge. The overall connectivity is therefore R-S-S-R. The terminology is widely used in biochemistry...
. The same mechanism can be used to bind two receptors together to form a homo- or heterodimer. The transmembrane element is a single α helix. The intracellular or cytoplasmic domain is responsible for the (highly conserved) kinase activity, as well as several regulatory functions.
Regulation
Ligand binding causes two reactions:- DimerProtein dimerIn biochemistry, a dimer is a macromolecular complex formed by two, usually non-covalently bound, macromolecules like proteins or nucleic acids...
ization of two monomeric receptor kinases or stabilization of a loose dimer. Many ligands of receptor tyrosine kinases are multivalentValence (chemistry)In chemistry, valence, also known as valency or valence number, is a measure of the number of bonds formed by an atom of a given element. "Valence" can be defined as the number of valence bonds...
. Some tyrosine receptor kinases (e.g., the platelet-derived growth factorPlatelet-derived growth factorIn molecular biology, platelet-derived growth factor is one of the numerous growth factors, or proteins that regulate cell growth and division. In particular, it plays a significant role in blood vessel formation , the growth of blood vessels from already-existing blood vessel tissue. Uncontrolled...
receptor) can form heterodimers with other similar but not identical kinases of the same subfamily, allowing a highly varied response to the extracellular signal. - Trans-autophosphorylation (phosphorylation by the other kinase in the dimer or higher order multimer complex) of the kinase.
The autophosphorylation causes the two subdomains of the intrinsic kinase to shift, opening the kinase domain for ATP binding. In the inactive form, the kinase subdomains are aligned so that ATP cannot reach the catalytic center of the kinase. When several amino acids suitable for phosphorylation are present in the kinase domain (e.g., the insulin-like growth factor receptor), the activity of the kinase can increase with the number of phosphorylated amino acids; in this case, the first phosphorylation is said to be a cis-autophosphorylation, switching the kinase from "off" to "standby".
Signal transduction
The active tyrosine kinase phosphorylates specific target proteins, which are often enzymes themselves. An important target is the ras protein signal-transduction chain.Receptor-associated tyrosine kinases
Tyrosine kinases recruited to a receptor following hormone binding are receptor-associated tyrosine kinases and are involved in a number of signaling cascades, in particular those involved in cytokineCytokine
Cytokines are small cell-signaling protein molecules that are secreted by the glial cells of the nervous system and by numerous cells of the immune system and are a category of signaling molecules used extensively in intercellular communication...
signaling (but also others, including growth hormone
Growth hormone
Growth hormone is a peptide hormone that stimulates growth, cell reproduction and regeneration in humans and other animals. Growth hormone is a 191-amino acid, single-chain polypeptide that is synthesized, stored, and secreted by the somatotroph cells within the lateral wings of the anterior...
). One such receptor-associated tyrosine kinase is Janus kinase
Janus kinase
Janus kinase is a family of intracellular, non-receptor tyrosine kinases that transduce cytokine-mediated signals via the JAK-STAT pathway. They were initially named "just another kinase" 1 & 2 , but were ultimately published as "Janus kinase"...
(JAK), many of whose effects are mediated by STAT protein
STAT protein
The STAT protein regulates many aspects of growth, survival and differentiation in cells...
s. (See JAK-STAT pathway.)
Histidine-specific protein kinases
Histidine kinaseHistidine kinase
Histidine Kinases are multifunctional, typically transmembrane, proteins of the transferase class that play a role in signal transduction across the cellular membrane. The vast majority of HKs are homodimers that exhibit autokinase, phosphotransfer, and phosphatase activity. HKs can act as...
s are structurally distinct from most other protein kinases and are found mostly in prokaryote
Prokaryote
The prokaryotes are a group of organisms that lack a cell nucleus , or any other membrane-bound organelles. The organisms that have a cell nucleus are called eukaryotes. Most prokaryotes are unicellular, but a few such as myxobacteria have multicellular stages in their life cycles...
s as part of two-component signal transduction mechanisms. A phosphate group from ATP is first added to a histidine residue within the kinase, and later transferred to an aspartate residue on a 'receiver domain' on a different protein, or sometimes on the kinase itself. The aspartyl phosphate residue is then active in signaling.
Histidine kinases are also found in plants, fungi and eukaryotes. The pyruvate dehydrogenase
Pyruvate dehydrogenase
Pyruvate dehydrogenase complex is a complex of three enzymes that transform pyruvate into acetyl-CoA by a process called pyruvate decarboxylation. Acetyl-CoA may then be used in the citric acid cycle to carry out cellular respiration, and this complex links the glycolysis metabolic pathway to the...
family of kinases in animals is structurally related to histidine kinases, but instead phosphorylate serine residues, and probably do not use a phospho-histidine intermediate.
Mixed kinases
Some kinases have mixed kinase activities. For example, MEK (MAPKK), which is involved in the MAP kinase cascade, is a mixed serine/threonine and tyrosine kinase and, hence, is a dual-specificity kinaseDual-specificity kinase
In biochemistry, a dual-specificity kinase is a kinase that can act as both tyrosine kinase and serine/threonine kinase.MEKs, involved in MAP pathways, are principal examples of dual-specificity kinases...
.
Inhibitors
Deregulated kinase activity is a frequent cause of disease, in particular cancer, wherein kinases regulate many aspects that control cell growth, movement and death. Drugs that inhibit specific kinases are being developed to treat several diseases, and some are currently in clinical use, including Gleevec (imatinibImatinib
Imatinib is a drug used to treat certain types of cancer. It is currently marketed by Novartis as Gleevec or Glivec as its mesylate salt, imatinib mesilate . It is used in treating chronic myelogenous leukemia , gastrointestinal stromal tumors and some other diseases...
) and Iressa (gefitinib
Gefitinib
Gefitinib INN , trade name Iressa, is a drug used in the treatment of certain types of cancer, particularly those with mutated and overactive EGFR. Gefitinib is an EGFR inhibitor, like erlotinib, which interrupts signaling through the epidermal growth factor receptor in target cells...
).
- Anthra(1,9-cd)pyrazol-6(2H)-oneAnthra(1,9-cd)pyrazol-6(2H)-one1,9-Pyrazoloanthrone is a chemical compound that is a derivative of anthrone. It is used in biochemical studies as an inhbitor of c-Jun N-terminal kinases .Derivatives of 1,9-pyrazoloanthrone have a variety of biological activities...
- StaurosporineStaurosporineStaurosporine is a natural product originally isolated in 1977 from the bacterium Streptomyces staurosporeus.It was the first of over 50 alkaloids to be isolated with this type of bis-indole chemical structure...
Kinase assays and profiling
Drug developments for kinase inhibitors are started from kinase assays, the lead compounds are usually profiled for specificity before moving into further tests. Many profiling services are available from fluorescent-based assays like the microscale thermophoresisMicroscale Thermophoresis
Microscale Thermophoresis is a technology for the analysis of biomolecules. Microscale Thermophoresis is the directed movement of particles in a microscopic temperature gradient...
assay to radioisotope based detections, and competition binding assays.
External links
- The Protein Kinase Resource: (new link!)Curated database of protein kinase structures and related data
- Human and mouse protein kinases: classification and index
- The Kinase Knowledgebase (KKB): Database of kinase structure-activity and chemical synthesis data.
- Kinase.Com: Genomics, evolution and large-scale analysis of protein kinases (non-commercial).
- Kinase/TIP: Database containing thousands of protein structures, co-complexes and models surveying the Human Kinome.
- AurSCOPE Kinase Database
- Kinasecentral: Information on Kinase inhibitors in development
- Collection of Ser/Thr/Tyr specific protein kinases and similar sequences
- KinMutBase: A registry of disease-causing mutations in protein kinase domains
- Human kinome by Manning et al. - Orientations of C1 domains of protein kinases in membranes - Orientations of C2 domains of protein kinases and other proteins in membranes
- Huaxian Chen, et.al. A Cell Based Immunochemical Assay for Monitoring Kinase Signaling Pathways and Drug Efficacy (PDF) Analytical Biochemistry 338 (2005) 136-142
- MAP Kinase Resource

