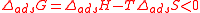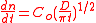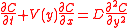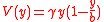Protein adsorption
Encyclopedia
Adsorption
(not to be mistaken for absorption) is the accumulation and adhesion of molecules, atoms, ions, or larger particles to a surface, but without actually penetrating the surface. The adsorption of larger biomolecules such as proteins is of high physiological relevance, and adsorb with different mechanisms than their molecular or atomic analogs. Some of the major driving forces behind protein adsorption include: surface energy, intermolecular forces, hydrophobicity, and ionic or electrostatic interaction. By knowing how these factors affect protein adsorption, they can then be manipulated by machining, alloying, and other engineering techniques to select for the most optimal performance in biomedical or physiological applications.
, the body will no longer be exposed to the foreign material, and will stop the immune response.
Proteins such as collagen
or fibrin
often serve as scaffolds for cell adhesion and cell growth. This is an integral part to the structural integrity of cell sheets and their differentiation into more complex tissue and organ structures. The adhesion properties of proteins to non-biological surfaces greatly influences whether or not cells can indirectly attach to them via scaffolds. An implant like a hip-stem replacement necessitates integration with the host tissues, and protein adsorption facilitates this integration.
Surgical tools can be designed to be sterilized more easily so that proteins do not remain adsorbed to a surface, risking cross-contamination. Some diseases such as Creutzfeldt–Jakob disease and kuru
(both related to mad cow disease) are caused by the transmission of prion
s, which are errant or improperly folded forms of a normally native protein. Surgical tools contaminated with prions require a special method of sterilization to completely eradicate all trace elements of the misfolded protein, as they are resistant to many of the normally used cleansing methods.
However, in some cases, protein adsorption to biomaterials can be an extremely unfavorable event. The adhesion of clotting factors may induce thrombosis
, which may lead to stroke
or other blockages. Some devices are intended to interact with the internal body environment such as sensors or drug-delivery vehicles, and protein adsorption would hinder their effectiveness.
subunits. Each amino acid has a side chain that gains or loses charge depending on the pH of the surrounding environment, as well as its own individual polar/nonpolar qualities. Charged regions can greatly contribute to how that protein interacts with other molecules and surfaces, as well as its own tertiary structure (protein folding). As a result of their hydrophilicity, charged amino acids tend to be located on the outside of proteins, where they are able to interact with surfaces. It is the unique combination of amino acids that gives a protein its properties. In terms of surface chemistry
, protein adsorption
is a critical phenomenon that describes the aggregation of these molecules on the exterior of a material. The tendency for proteins to remain attached to a surface depends largely on the material properties such as surface energy, texture, and relative charge distribution. Larger proteins are more likely to adsorb and remain attached to a surface due to the higher number of contact sites between amino acids and the surface (Figure 1).
This is seen in the equation:
where:
In order for the protein adsorption to occur spontaneously, ∆adsG must be a negative number.
of its amino acid
side chains, and the terminal amino acid and carboxylic acid. Groups with pHs above physiologic con
ditions have a positive charge and groups with pH below have a negative charge. The net charge of the protein, determined by the sum charge it's constituents, results in electrophoretic migration in a physiologic electric field. These effects are short-range because of the high di-electric constant of water, however, once the protein is close to a charged surface, electrostatic coupling becomes the dominant force.
, thermal convection, bulk flow, or a combination thereof. When considering the transport of proteins, it is clear how concentration gradients, temperature, protein size and flow velocity will influence the arrival of proteins to a solid surface. Under conditions of low flow and minimal temperature gradients, the adsorption rate can be modeled after the diffusion rate equation.
where:
A higher bulk concentration and/or higher diffusion coefficient (inversely proportional to molecular size) results in a larger number of molecules arriving at the surface. The consequential protein surface interactions result in high local concentrations of adsorbed protein, reaching concentrations of up to 1000 times higher than in the bulk solution. However, the body is much more complex, containing flow and convective diffusion, and these must be considered in the rate of protein adsorption.
and
where:
This equation is especially applicable to analyzing protein adsorption to biomedical devices in arteries, e.g. stents.
ing refers to the specific bonding between positive metal ions and surrounding valence electron clouds. This intermolecular force is relatively strong, and gives rise to the repeated crystal
line orientation of atoms, also referred to as its lattice system. There are several types of common lattice formations, and each has its own unique packing density and atomic closeness. The negatively charged electron clouds of the metal ions will sterically hinder the adhesion of negatively charged protein regions due to charge repulsion
, thus limiting the available binding sites of a protein to a metal surface.
The lattice formation can lead to connection with exposed potential metal-ion-dependent adhesion sites (MIDAS) which are binding sites for collagen and other proteins. The surface of the metal has different properties than the bulk since the normal crystalline repeating subunits is terminated at the surface. This leaves the surface atoms without a neighboring atom on one side, which inherently alters the electron distribution. This phenomenon also explains why the surface atoms have a higher energy than the bulk, often simply referred to as surface energy
. This state of higher energy is unfavorable, and the surface atoms will try to reduce it by binding to available reactive molecules. This is often accomplished by protein adsorption, where the surface atoms are reduced to a more advantageous energy state.
The internal environment of the body is often modeled to be an aqueous environment at 37°C at pH 7.3 with plenty of dissolved oxygen, electrolytes, proteins, and cells. When exposed to oxygen for an extended period of time, many metals may become oxidized
and increase their surface oxidation state
by losing electrons. This new cationic state leaves the surface with a net positive charge, and a higher affinity for negatively charged protein side groups. Within the vast diversity of metals and metal alloys, many are susceptible to corrosion when implanted in the body. Elements that are more electronegative are corroded faster when exposed to an electrolyte-rich aqueous environment such as the human body. Both oxidation and corrosion will lower the free energy, thus affecting protein adsorption as seen in Eq. 1.
techniques are often used to increase protein adhesion to implants in the hopes of shortening recovery time. The technique of laserpatterning introduces grooves and surface roughness that will influence adhesion, migration and alignment. Grit-blasting, a method analogous to sand blasting, and chemical etching have proven to be successful surface roughening techniques that promote the long-term stability of titanium implants. The increase in stability is a direct result of the observed increase in extracellular matrix and collagen attachment, which results in increased osteoblast attachment and mineralization when compared to non-roughened surfaces. Adsorption is not always desirable, however. Machinery can be negatively affected by adsorption, particularly with Protein adsorption in the food industry
.
s are of great importance when considering protein adsorption in the biomedical arena. Polymers are composed of one or more types of "mers" bound together repeatedly, typically by directional covalent bonds. As the chain grows by the addition of mers, the chemical and physical properties of the material are dictated by the molecular structure of the monomer. By carefully selecting the type or types of mers in a polymer and its manufacturing process, the chemical and physical properties of a polymer can be highly tailored to adsorb specific proteins and cells for a particular application.
, tertiary
, and quartary structures of proteins. In addition to adsorption rates and amounts, orientation and conformation are of critical importance. These conformational changes can affect protein interaction with ligand
s, substrates, and antigen
s which are dependent on the orientation of the binding site of interest. These conformational changes, as a result of protein adsorption, can also denature
the protein and change its native properties.
is a relatively new field that utilizes a scaffolding
as a platform upon which the desired cells proliferate. It is not clear what defines an ideal scaffold for a specific tissue type. The considerations are complex and protein adsorption only adds to the complexity. Although architecture, structural mechanics, and surface properties play a key role, understanding degradation and rate of protein adsorption are also key. In addition to the essentials of mechanics and geometry, a suitable scaffold construct will possess surface properties that are optimized for the attachment and migration of the cell types of particular interest.
Generally, it has been found that scaffolds that closely resemble the natural environments of the tissue being engineered are the most successful. As a result, much research has gone into investigating natural polymers that can be tailored, through processing methodology, toward specific design criteria. Chitosan
is currently one of the most widely used polymers as it is very similar to naturally occurring glycosaminoglycan
(GAGs) and it is degradable by human enzymes.
Table 1: Structures, target tissues, and application cell types of chitosan-based scaffolds
Adsorption
Adsorption is the adhesion of atoms, ions, biomolecules or molecules of gas, liquid, or dissolved solids to a surface. This process creates a film of the adsorbate on the surface of the adsorbent. It differs from absorption, in which a fluid permeates or is dissolved by a liquid or solid...
(not to be mistaken for absorption) is the accumulation and adhesion of molecules, atoms, ions, or larger particles to a surface, but without actually penetrating the surface. The adsorption of larger biomolecules such as proteins is of high physiological relevance, and adsorb with different mechanisms than their molecular or atomic analogs. Some of the major driving forces behind protein adsorption include: surface energy, intermolecular forces, hydrophobicity, and ionic or electrostatic interaction. By knowing how these factors affect protein adsorption, they can then be manipulated by machining, alloying, and other engineering techniques to select for the most optimal performance in biomedical or physiological applications.
Relevance
Many medical devices and products come into contact with the internal surfaces of the body, such as surgical tools and implants. When a non-native material enters the body, the first step of the immune response takes place and host extracellular matrix and plasma proteins aggregate to the material in attempts to contain, neutralize, or wall-off the injurious agent. These proteins can facilitate the attachment of various cell types such as osteoblasts and fibroblasts that can encourage tissue repair. Taking this a step further, implantable devices can be coated with a bioactive material to encourage adsorption of specific proteins, fibrous capsule formation, and wound healing. This would reduce the risk of implant rejection and accelerate recovery by selecting for the necessary proteins and cells necessary for endothelialization. After the formation of the endotheliumEndothelium
The endothelium is the thin layer of cells that lines the interior surface of blood vessels, forming an interface between circulating blood in the lumen and the rest of the vessel wall. These cells are called endothelial cells. Endothelial cells line the entire circulatory system, from the heart...
, the body will no longer be exposed to the foreign material, and will stop the immune response.
Proteins such as collagen
Collagen
Collagen is a group of naturally occurring proteins found in animals, especially in the flesh and connective tissues of mammals. It is the main component of connective tissue, and is the most abundant protein in mammals, making up about 25% to 35% of the whole-body protein content...
or fibrin
Fibrin
Fibrin is a fibrous, non-globular protein involved in the clotting of blood. It is a fibrillar protein that is polymerised to form a "mesh" that forms a hemostatic plug or clot over a wound site....
often serve as scaffolds for cell adhesion and cell growth. This is an integral part to the structural integrity of cell sheets and their differentiation into more complex tissue and organ structures. The adhesion properties of proteins to non-biological surfaces greatly influences whether or not cells can indirectly attach to them via scaffolds. An implant like a hip-stem replacement necessitates integration with the host tissues, and protein adsorption facilitates this integration.
Surgical tools can be designed to be sterilized more easily so that proteins do not remain adsorbed to a surface, risking cross-contamination. Some diseases such as Creutzfeldt–Jakob disease and kuru
Kuru (disease)
Kuru is an incurable degenerative neurological disorder that is a type of transmissible spongiform encephalopathy, caused by a prion found in humans...
(both related to mad cow disease) are caused by the transmission of prion
Prion
A prion is an infectious agent composed of protein in a misfolded form. This is in contrast to all other known infectious agents which must contain nucleic acids . The word prion, coined in 1982 by Stanley B. Prusiner, is a portmanteau derived from the words protein and infection...
s, which are errant or improperly folded forms of a normally native protein. Surgical tools contaminated with prions require a special method of sterilization to completely eradicate all trace elements of the misfolded protein, as they are resistant to many of the normally used cleansing methods.
However, in some cases, protein adsorption to biomaterials can be an extremely unfavorable event. The adhesion of clotting factors may induce thrombosis
Thrombosis
Thrombosis is the formation of a blood clot inside a blood vessel, obstructing the flow of blood through the circulatory system. When a blood vessel is injured, the body uses platelets and fibrin to form a blood clot to prevent blood loss...
, which may lead to stroke
Stroke
A stroke, previously known medically as a cerebrovascular accident , is the rapidly developing loss of brain function due to disturbance in the blood supply to the brain. This can be due to ischemia caused by blockage , or a hemorrhage...
or other blockages. Some devices are intended to interact with the internal body environment such as sensors or drug-delivery vehicles, and protein adsorption would hinder their effectiveness.
Fundamentals of Protein Adsorption
Proteins are small biomolecules that can be composed of amino acidAmino acid
Amino acids are molecules containing an amine group, a carboxylic acid group and a side-chain that varies between different amino acids. The key elements of an amino acid are carbon, hydrogen, oxygen, and nitrogen...
subunits. Each amino acid has a side chain that gains or loses charge depending on the pH of the surrounding environment, as well as its own individual polar/nonpolar qualities. Charged regions can greatly contribute to how that protein interacts with other molecules and surfaces, as well as its own tertiary structure (protein folding). As a result of their hydrophilicity, charged amino acids tend to be located on the outside of proteins, where they are able to interact with surfaces. It is the unique combination of amino acids that gives a protein its properties. In terms of surface chemistry
Surface science
Surface science is the study of physical and chemical phenomena that occur at the interface of two phases, including solid–liquid interfaces, solid–gas interfaces, solid–vacuum interfaces, and liquid-gas interfaces. It includes the fields of surface chemistry and surface physics. Some related...
, protein adsorption
Adsorption
Adsorption is the adhesion of atoms, ions, biomolecules or molecules of gas, liquid, or dissolved solids to a surface. This process creates a film of the adsorbate on the surface of the adsorbent. It differs from absorption, in which a fluid permeates or is dissolved by a liquid or solid...
is a critical phenomenon that describes the aggregation of these molecules on the exterior of a material. The tendency for proteins to remain attached to a surface depends largely on the material properties such as surface energy, texture, and relative charge distribution. Larger proteins are more likely to adsorb and remain attached to a surface due to the higher number of contact sites between amino acids and the surface (Figure 1).
Energy of Protein Adsorption
The fundamental idea behind spontaneous protein adsorption is that adsorption occurs when more energy is released than gained according to Gibbs law of free energy.This is seen in the equation:
where:
- ∆ads is net change of the parameters
- G is Gibbs free energyGibbs free energyIn thermodynamics, the Gibbs free energy is a thermodynamic potential that measures the "useful" or process-initiating work obtainable from a thermodynamic system at a constant temperature and pressure...
- T is the temperatureTemperatureTemperature is a physical property of matter that quantitatively expresses the common notions of hot and cold. Objects of low temperature are cold, while various degrees of higher temperatures are referred to as warm or hot...
(SI unit: kelvinKelvinThe kelvin is a unit of measurement for temperature. It is one of the seven base units in the International System of Units and is assigned the unit symbol K. The Kelvin scale is an absolute, thermodynamic temperature scale using as its null point absolute zero, the temperature at which all...
) - S is the entropyEntropyEntropy is a thermodynamic property that can be used to determine the energy available for useful work in a thermodynamic process, such as in energy conversion devices, engines, or machines. Such devices can only be driven by convertible energy, and have a theoretical maximum efficiency when...
(SI unit: joule per kelvin) - H is the enthalpyEnthalpyEnthalpy is a measure of the total energy of a thermodynamic system. It includes the internal energy, which is the energy required to create a system, and the amount of energy required to make room for it by displacing its environment and establishing its volume and pressure.Enthalpy is a...
(SI unit: joule)
In order for the protein adsorption to occur spontaneously, ∆adsG must be a negative number.
Vroman Effect
Proteins and other molecules are constantly in competition with one another over binding sites on a surface. The Vroman Effect, developed by Leo Vroman, postulates that small and abundant molecules will be the first to coat a surface. However, over time, molecules with higher affinity for that particular surface will replace them. This is often seen in materials that contact the blood where fibrin, which is usually abundant, will bind to the surface first and over time will be replaced by larger proteins.Forces and Interactions
The four fundamental classes of forces and interaction in protein adsorption are: 1) ionic or electrostatic interaction, 2) hydrogen bonding, 3) hydrophobic interaction (largely entropically driven), and 4) interactions of charge-transfer or particle electron donor/acceptor type.Ionic or Electrostatic Interactions
The charge of proteins is determined by the pKaPKA
PKA, pKa, or other similar variations may stand for:* pKa, the symbol for the acid dissociation constant at logarithmic scale* Protein kinase A, a class of cAMP-dependent enzymes* Pi Kappa Alpha, the North-American social fraternity...
of its amino acid
Amino acid
Amino acids are molecules containing an amine group, a carboxylic acid group and a side-chain that varies between different amino acids. The key elements of an amino acid are carbon, hydrogen, oxygen, and nitrogen...
side chains, and the terminal amino acid and carboxylic acid. Groups with pHs above physiologic con
ditions have a positive charge and groups with pH below have a negative charge. The net charge of the protein, determined by the sum charge it's constituents, results in electrophoretic migration in a physiologic electric field. These effects are short-range because of the high di-electric constant of water, however, once the protein is close to a charged surface, electrostatic coupling becomes the dominant force.
Hydrogen Bonding
Water has as much propensity to form hydrogen bonds as any group in a polypeptide. During a folding and association process, peptide and amino acid groups exchange hydrogen bonds with water. Thus, hydrogen bonding does not have a strong stabilizing effect on protein adsorption in an aqueous medium.Hydrophobic Interactions
Hydrophobic interactions are essentially entropic interactions basically due to order/disorder phenomena in an aqueous medium. The free energy associated with minimizing interfacial areas is responsible for minimizing the surface area of water droplets and air bubbles in water. This same principle is the reason that hydrophobic amino acid side chains are oriented away from water, minimizing their interaction with water. The hydrophilic groups on the outside of the molecule result in protein water solubility. Characterizing this phenomenon can be done by treating these hydrophobic relationships with interfacial free energy concepts. Accordingly, one can think of the driving force of these interactions as the minimization of total interfacial free energy, i.e. minimization of surface area.Charge-Transfer Interactions
Charge-transfer interactions are also important in protein stabilization and surface interaction. In general donor-acceptor processes, one can think of excess electron density being present which can be donated to an electrophilic species. In aqueous media, these solute interactions are primarily due to pi orbital electron effects.Rate of Adsorption
In order for proteins to adsorb, they must first come into contact with the surface through one or more of these major transport mechanisms: diffusionDiffusion
Molecular diffusion, often called simply diffusion, is the thermal motion of all particles at temperatures above absolute zero. The rate of this movement is a function of temperature, viscosity of the fluid and the size of the particles...
, thermal convection, bulk flow, or a combination thereof. When considering the transport of proteins, it is clear how concentration gradients, temperature, protein size and flow velocity will influence the arrival of proteins to a solid surface. Under conditions of low flow and minimal temperature gradients, the adsorption rate can be modeled after the diffusion rate equation.
Diffusion Rate equation
where:
- D is the diffusion coefficient
- n is the surface concentration of protein
- Co is the bulk concentration of proteins
- t is time
A higher bulk concentration and/or higher diffusion coefficient (inversely proportional to molecular size) results in a larger number of molecules arriving at the surface. The consequential protein surface interactions result in high local concentrations of adsorbed protein, reaching concentrations of up to 1000 times higher than in the bulk solution. However, the body is much more complex, containing flow and convective diffusion, and these must be considered in the rate of protein adsorption.
Flow in a thin channel
and
where:
- C is concentration
- D is the diffusion coefficient
- V is the velocity of flow
- x is the distance down the channel
- γ is the wall shear rate
- b is the height of the channel
This equation is especially applicable to analyzing protein adsorption to biomedical devices in arteries, e.g. stents.
Chemical composition
Metallic bondMetallic bond
Metallic bonding is the electrostatic attractive forces between the delocalized electrons, called conduction electrons, gathered in an "electron sea", and the positively charged metal ions...
ing refers to the specific bonding between positive metal ions and surrounding valence electron clouds. This intermolecular force is relatively strong, and gives rise to the repeated crystal
Crystal
A crystal or crystalline solid is a solid material whose constituent atoms, molecules, or ions are arranged in an orderly repeating pattern extending in all three spatial dimensions. The scientific study of crystals and crystal formation is known as crystallography...
line orientation of atoms, also referred to as its lattice system. There are several types of common lattice formations, and each has its own unique packing density and atomic closeness. The negatively charged electron clouds of the metal ions will sterically hinder the adhesion of negatively charged protein regions due to charge repulsion
Electric charge
Electric charge is a physical property of matter that causes it to experience a force when near other electrically charged matter. Electric charge comes in two types, called positive and negative. Two positively charged substances, or objects, experience a mutual repulsive force, as do two...
, thus limiting the available binding sites of a protein to a metal surface.
The lattice formation can lead to connection with exposed potential metal-ion-dependent adhesion sites (MIDAS) which are binding sites for collagen and other proteins. The surface of the metal has different properties than the bulk since the normal crystalline repeating subunits is terminated at the surface. This leaves the surface atoms without a neighboring atom on one side, which inherently alters the electron distribution. This phenomenon also explains why the surface atoms have a higher energy than the bulk, often simply referred to as surface energy
Surface energy
Surface energy quantifies the disruption of intermolecular bonds that occur when a surface is created. In the physics of solids, surfaces must be intrinsically less energetically favorable than the bulk of a material, otherwise there would be a driving force for surfaces to be created, removing...
. This state of higher energy is unfavorable, and the surface atoms will try to reduce it by binding to available reactive molecules. This is often accomplished by protein adsorption, where the surface atoms are reduced to a more advantageous energy state.
The internal environment of the body is often modeled to be an aqueous environment at 37°C at pH 7.3 with plenty of dissolved oxygen, electrolytes, proteins, and cells. When exposed to oxygen for an extended period of time, many metals may become oxidized
Redox
Redox reactions describe all chemical reactions in which atoms have their oxidation state changed....
and increase their surface oxidation state
Oxidation state
In chemistry, the oxidation state is an indicator of the degree of oxidation of an atom in a chemical compound. The formal oxidation state is the hypothetical charge that an atom would have if all bonds to atoms of different elements were 100% ionic. Oxidation states are typically represented by...
by losing electrons. This new cationic state leaves the surface with a net positive charge, and a higher affinity for negatively charged protein side groups. Within the vast diversity of metals and metal alloys, many are susceptible to corrosion when implanted in the body. Elements that are more electronegative are corroded faster when exposed to an electrolyte-rich aqueous environment such as the human body. Both oxidation and corrosion will lower the free energy, thus affecting protein adsorption as seen in Eq. 1.
Effects of Machining
Surface roughness and texture has an undeniable influence on protein adsorption on all materials, but with the ubiquity of metal machining processes, it is useful to address how these impact protein behavior. The initial adsorption is important, as well as maintained adhesion and integrity. Research has shown that surface roughness can encourage the adhesion of scaffold proteins and osteoblasts, and results in an increase in surface mineralization. Surfaces with more topographical features and roughness will have more exposed surface area for proteins to interact with. In terms of biomedical engineering applications, micromachiningSurface micromachining
Unlike Bulk micromachining, where a silicon substrate is selectively etched to produce structures, surface micromachining builds microstructures by deposition and etching of different structural layers on top of the substrate....
techniques are often used to increase protein adhesion to implants in the hopes of shortening recovery time. The technique of laserpatterning introduces grooves and surface roughness that will influence adhesion, migration and alignment. Grit-blasting, a method analogous to sand blasting, and chemical etching have proven to be successful surface roughening techniques that promote the long-term stability of titanium implants. The increase in stability is a direct result of the observed increase in extracellular matrix and collagen attachment, which results in increased osteoblast attachment and mineralization when compared to non-roughened surfaces. Adsorption is not always desirable, however. Machinery can be negatively affected by adsorption, particularly with Protein adsorption in the food industry
Protein adsorption in the food industry
Protein adsorption refers to the adhesion of proteins to solid surfaces. This phenomenon is an important issue in the food processing industry, particularly in milk processing and wine and beer making. Excessive adsorption, or protein fouling, can lead to health and sanitation issues, as the...
.
Protein adsorption to polymers
PolymerPolymer
A polymer is a large molecule composed of repeating structural units. These subunits are typically connected by covalent chemical bonds...
s are of great importance when considering protein adsorption in the biomedical arena. Polymers are composed of one or more types of "mers" bound together repeatedly, typically by directional covalent bonds. As the chain grows by the addition of mers, the chemical and physical properties of the material are dictated by the molecular structure of the monomer. By carefully selecting the type or types of mers in a polymer and its manufacturing process, the chemical and physical properties of a polymer can be highly tailored to adsorb specific proteins and cells for a particular application.
Conformation effects
Protein adsorption often results in significant conformational changes, which refers to changes in the secondarySecondary
Secondary is an adjective meaning "second" or "second hand". It may refer to:* The group of defensive backs in American football and Canadian football* An obsolete name for the Mesozoic in geosciences...
, tertiary
Tertiary
The Tertiary is a deprecated term for a geologic period 65 million to 2.6 million years ago. The Tertiary covered the time span between the superseded Secondary period and the Quaternary...
, and quartary structures of proteins. In addition to adsorption rates and amounts, orientation and conformation are of critical importance. These conformational changes can affect protein interaction with ligand
Ligand
In coordination chemistry, a ligand is an ion or molecule that binds to a central metal atom to form a coordination complex. The bonding between metal and ligand generally involves formal donation of one or more of the ligand's electron pairs. The nature of metal-ligand bonding can range from...
s, substrates, and antigen
Antigen
An antigen is a foreign molecule that, when introduced into the body, triggers the production of an antibody by the immune system. The immune system will then kill or neutralize the antigen that is recognized as a foreign and potentially harmful invader. These invaders can be molecules such as...
s which are dependent on the orientation of the binding site of interest. These conformational changes, as a result of protein adsorption, can also denature
Denaturation (biochemistry)
Denaturation is a process in which proteins or nucleic acids lose their tertiary structure and secondary structure by application of some external stress or compound, such as a strong acid or base, a concentrated inorganic salt, an organic solvent , or heat...
the protein and change its native properties.
Adsorption to polymer scaffolds
Tissue engineeringTissue engineering
Tissue engineering is the use of a combination of cells, engineering and materials methods, and suitable biochemical and physio-chemical factors to improve or replace biological functions...
is a relatively new field that utilizes a scaffolding
Scaffolding
Scaffolding is a temporary structure used to support people and material in the construction or repair of buildings and other large structures. It is usually a modular system of metal pipes or tubes, although it can be from other materials...
as a platform upon which the desired cells proliferate. It is not clear what defines an ideal scaffold for a specific tissue type. The considerations are complex and protein adsorption only adds to the complexity. Although architecture, structural mechanics, and surface properties play a key role, understanding degradation and rate of protein adsorption are also key. In addition to the essentials of mechanics and geometry, a suitable scaffold construct will possess surface properties that are optimized for the attachment and migration of the cell types of particular interest.
Generally, it has been found that scaffolds that closely resemble the natural environments of the tissue being engineered are the most successful. As a result, much research has gone into investigating natural polymers that can be tailored, through processing methodology, toward specific design criteria. Chitosan
Chitosan
Chitosan is a linear polysaccharide composed of randomly distributed β--linked D-glucosamine and N-acetyl-D-glucosamine...
is currently one of the most widely used polymers as it is very similar to naturally occurring glycosaminoglycan
Glycosaminoglycan
Glycosaminoglycans or mucopolysaccharides are long unbranched polysaccharides consisting of a repeating disaccharide unit. The repeating unit consists of a hexose or a hexuronic acid, linked to a hexosamine .-Production:Protein cores made in the rough endoplasmic reticulum are posttranslationally...
(GAGs) and it is degradable by human enzymes.
Chitosan
Chitosan is a linear polysaccharide containing linked chitin-derived residues and is widely studied as a biomaterial due to its high compatibility with numerous proteins in the body. Chitosan is cationic and thus electrostatically reacts with numerous proteoglycans, anionic GAGs, and other molecules possessing a negative charge. Since many cytokines and growth factors are linked to GAG, scaffolds with the chitosan-GAG complexes are able to retain these proteins secreted by the adhered cells. Another quality of chitosan that gives it good biomaterial potential is its high charge density in solutions. This allows chitosan to form ionic complexes with many water-soluble anionic polymers, expanding the range of proteins that are able to bind to it and thus expanding its possible uses.| Polymer | Scaffold structure | Target tissue | Application cell type | Ref |
|---|---|---|---|---|
| Chitosan | 3D porous blocks | Bone | Osteoblast-like ROS | |
| Chitosan-polyester | 3D fiber meshes | Bone | Human MSC | |
| Chitosan-alginate | Injectable gel | Bone | Osteoblast-like MG63 | |
| Chitosan-gelatin | 3D porous cylinders | Cartilage | Chondrocytes | |
| Chitosan-GP | Injectable gel | Cartilage | Chondrocytes | |
| Chitosan-collagen | Porous membranes | Skin | Fibroblast and keratinocyte co-culture |