
Principle of minimum energy
Encyclopedia
The principle of minimum energy is essentially a restatement of the second law of thermodynamics
. It states that for a closed system
, with constant external parameters and entropy, the internal energy will decrease and approach a minimum value at equilibrium. External parameters generally means the volume, but may include other parameters which are specified externally, such as a constant magnetic field.
In contrast, the second law states that for isolated system
s, (and fixed external parameters) the entropy
will increase to a maximum value at equilibrium. An isolated system
has a fixed total energy and mass. A closed system
, on the other hand, is a system which is connected to another system, and may exchange energy, but not mass, with the other system. If, rather than an isolated system, we have a closed system, in which the entropy rather than the energy remains constant, then it follows from the first and second laws of thermodynamics that the energy of that system will drop to a minimum value at equilibrium, transferring its energy to the other system. To restate:
This should not be confused with the minimum total potential energy principle
which states that, at equilibrium, the total potential energy of a system with dissipation will be at a minimum, which is a special case of the maximum entropy principle.
As an example, consider the familiar example of a marble on the edge of a bowl. If we consider the marble and bowl to be an isolated system, then when the marble drops, the potential energy will be converted to the kinetic energy
of motion of the marble. Frictional forces will convert this kinetic energy to heat, and at equilbrium, the marble will be at rest at the bottom of the bowl, and the marble and the bowl will be at a slightly higher temperature. The total energy of the marble-bowl system will be unchanged. What was previously the potential energy of the marble, will now reside in the increased heat energy of the marble-bowl system. This will be an application of the maximum entropy principle as set forth in the principle of minimum potential energy, since due to the heating effects, the entropy has increased to the maximum value possible given the fixed energy of the system.
If, on the other hand, the marble is lowered very slowly to the bottom of the bowl, so slowly that no heating effects occur (i.e. reversibly), then the entropy of the marble and bowl will remain constant, and the potential energy of the marble will be transferred as work energy to the apparatus that is lowering the marble. Since the potential energy is now at a minimum with no increase in the energy due to heat of either the marble or the bowl, the total energy of the system is at a minimum. This is an application of the minimum energy principle.
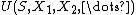 where S is entropy, and the
where S is entropy, and the 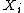 are the other extensive parameters of the system (e.g. volume, particle number
are the other extensive parameters of the system (e.g. volume, particle number
, etc.). The entropy of the system may likewise be written as a function of the other extensive parameters as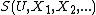 . Suppose that X is one of the
. Suppose that X is one of the 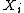 which varies as a system approaches equilibrium, and that it is the only such parameter which is varying. The principle of maximum entropy may then be stated as:
which varies as a system approaches equilibrium, and that it is the only such parameter which is varying. The principle of maximum entropy may then be stated as:
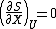 and
and 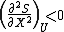 at equilibrium.
at equilibrium.
The first condition states that entropy is at an extremum, and the second condition states that entropy is at a maximum. Note that for the partial derivatives, all extensive parameters are assumed constant except for the variables contained in the partial derivative, but only U, S, or X are shown. It follows from the properties of an exact differential (see equation 7 in the exact differential
article) and from the energy/entropy equation of state that, for a closed system:
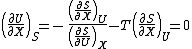
It is seen that the energy is at an extremum at equilibrium. By similar but somewhat more lengthy argument it can be shown that
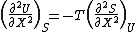
which is greater than zero, showing that the energy is, in fact, at a minimum. (See Callen (1985) chapter 5).
Suppose that x is smaller than its equilibrium value. The upward force of the gas is greater than the downward force of the weight, and if allowed to freely move, the gas in the cylinder would push the weight upward rapidly, and there would be frictional forces that would convert the energy to heat. If we specify that an external agent presses down on the weight so as to very slowly (reversibly) allow the weight to move upward to its equilibrium position, then there will be no heat generated and the entropy of the system will remain constant while energy is transferred as work to the external agent. The total energy of the system at any value of x is given by the internal energy of the gas plus the potential energy of the weight:
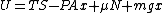
where T is temperature, S is entropy, P is pressure, μ is the chemical potential, N is the number of particles in the gas, and the volume has been written as V=Ax. Since the system is closed, the particle number N is constant and a small change in the energy of the system would be given by:
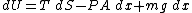
Since the entropy is constant, we may say that dS=0 at equilibrium and by the principle of minimum energy, we may say that dU=0 at equilibrium, yielding the equilibrium condition:
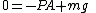
which simply states that the upward gas pressure force (PA) on the upper face of the cylinder is equal to the downward force of the mass due to gravitation (mg).
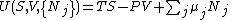
where the intensive parameters (T, P, μj) are functions of the internal energy's natural variables via the equations of state. As an example of another thermodynamic potential, the Helmholtz free energy
via the equations of state. As an example of another thermodynamic potential, the Helmholtz free energy
is written:
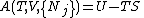
where temperature has replaced entropy as a natural variable. In order to understand the value of the thermodynamic potentials, it is necessary to view them in a different light. They may in fact be seen as (negative) Legendre transforms of the internal energy, in which certain of the extensive parameters are replaced by the derivative of internal energy with respect to that variable (i.e. the conjugate
to that variable). For example, the Helmholtz free energy may be written:
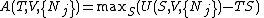
and the maximum will occur when the variable T becomes equal to the temperature since
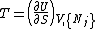
The Helmholtz free energy is a useful quantity when studying thermodynamic transformations in which the temperature is held constant. Although the reduction in the number of variables is a useful simplification, the main advantage comes from the fact that the Helmholtz free energy is minimized at equilibrium with respect to any unconstrained internal variables for a closed system
at constant temperature and volume. This follows directly from the principle of minimum energy which states that at constant entropy, the internal energy is minimized. This can be stated as:
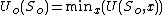
where and
and 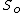 are the value of the internal energy and the (fixed) entropy at equilibrium. The volume and particle number variables have been replaced by x which stands for any internal unconstrained variables.
are the value of the internal energy and the (fixed) entropy at equilibrium. The volume and particle number variables have been replaced by x which stands for any internal unconstrained variables.
The minimization is with respect to the unconstrained variables. In the case of chemical reactions this is usually the number of particles or mole fractions, subject to the conservation of elements. At equilibrium, these will take on their equilibrium values, and the internal energy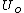 will be a function only of the chosen value of entropy
will be a function only of the chosen value of entropy 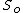 . By the definition of the Legendre transform, the Helmholtz free energy will be:
. By the definition of the Legendre transform, the Helmholtz free energy will be:
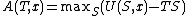
The Helmholtz free energy at equilibrium will be:
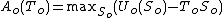
where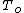 is the (unknown) temperature at equilibrium. Substituting the expression for
is the (unknown) temperature at equilibrium. Substituting the expression for 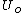 :
:
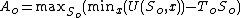
Assuming the order of the extrema can be exchanged:
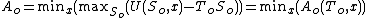
showing that the Helmholtz free energy is minimized at equilibrium.
The Enthalpy
and Gibbs free energy
, are similarly derived.
Second law of thermodynamics
The second law of thermodynamics is an expression of the tendency that over time, differences in temperature, pressure, and chemical potential equilibrate in an isolated physical system. From the state of thermodynamic equilibrium, the law deduced the principle of the increase of entropy and...
. It states that for a closed system
Closed system
-In physics:In thermodynamics, a closed system can exchange energy , but not matter, with its surroundings.In contrast, an isolated system cannot exchange any of heat, work, or matter with the surroundings, while an open system can exchange all of heat, work and matter.For a simple system, with...
, with constant external parameters and entropy, the internal energy will decrease and approach a minimum value at equilibrium. External parameters generally means the volume, but may include other parameters which are specified externally, such as a constant magnetic field.
In contrast, the second law states that for isolated system
Isolated system
In the natural sciences an isolated system, as contrasted with an open system, is a physical system without any external exchange. If it has any surroundings, it does not interact with them. It obeys in particular the first of the conservation laws: its total energy - mass stays constant...
s, (and fixed external parameters) the entropy
Entropy
Entropy is a thermodynamic property that can be used to determine the energy available for useful work in a thermodynamic process, such as in energy conversion devices, engines, or machines. Such devices can only be driven by convertible energy, and have a theoretical maximum efficiency when...
will increase to a maximum value at equilibrium. An isolated system
Isolated system
In the natural sciences an isolated system, as contrasted with an open system, is a physical system without any external exchange. If it has any surroundings, it does not interact with them. It obeys in particular the first of the conservation laws: its total energy - mass stays constant...
has a fixed total energy and mass. A closed system
Closed system
-In physics:In thermodynamics, a closed system can exchange energy , but not matter, with its surroundings.In contrast, an isolated system cannot exchange any of heat, work, or matter with the surroundings, while an open system can exchange all of heat, work and matter.For a simple system, with...
, on the other hand, is a system which is connected to another system, and may exchange energy, but not mass, with the other system. If, rather than an isolated system, we have a closed system, in which the entropy rather than the energy remains constant, then it follows from the first and second laws of thermodynamics that the energy of that system will drop to a minimum value at equilibrium, transferring its energy to the other system. To restate:
- The maximum entropy principle: For a closed system with fixed internal energy (i.e. an isolated system), the entropy is maximized at equilibrium.
- The minimum energy principle: For a closed system with fixed entropy, the total energy is minimized at equilibrium.
This should not be confused with the minimum total potential energy principle
Minimum total potential energy principle
The principle of minimum total potential energy is a fundamental concept used in physics, chemistry, biology, and engineering. It asserts that a structure or body shall deform or displace to a position that minimizes the total potential energy, with the lost potential energy being dissipated as heat...
which states that, at equilibrium, the total potential energy of a system with dissipation will be at a minimum, which is a special case of the maximum entropy principle.
As an example, consider the familiar example of a marble on the edge of a bowl. If we consider the marble and bowl to be an isolated system, then when the marble drops, the potential energy will be converted to the kinetic energy
Kinetic energy
The kinetic energy of an object is the energy which it possesses due to its motion.It is defined as the work needed to accelerate a body of a given mass from rest to its stated velocity. Having gained this energy during its acceleration, the body maintains this kinetic energy unless its speed changes...
of motion of the marble. Frictional forces will convert this kinetic energy to heat, and at equilbrium, the marble will be at rest at the bottom of the bowl, and the marble and the bowl will be at a slightly higher temperature. The total energy of the marble-bowl system will be unchanged. What was previously the potential energy of the marble, will now reside in the increased heat energy of the marble-bowl system. This will be an application of the maximum entropy principle as set forth in the principle of minimum potential energy, since due to the heating effects, the entropy has increased to the maximum value possible given the fixed energy of the system.
If, on the other hand, the marble is lowered very slowly to the bottom of the bowl, so slowly that no heating effects occur (i.e. reversibly), then the entropy of the marble and bowl will remain constant, and the potential energy of the marble will be transferred as work energy to the apparatus that is lowering the marble. Since the potential energy is now at a minimum with no increase in the energy due to heat of either the marble or the bowl, the total energy of the system is at a minimum. This is an application of the minimum energy principle.
Mathematical explanation
The total energy of the system is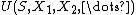 where S is entropy, and the
where S is entropy, and the 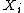 are the other extensive parameters of the system (e.g. volume, particle number
are the other extensive parameters of the system (e.g. volume, particle numberParticle number
The particle number of a thermodynamic system, conventionally indicated with the letter N, is the number of constituent particles in that system. The particle number is a fundamental parameter in thermodynamics which is conjugate to the chemical potential. Unlike most physical quantities, particle...
, etc.). The entropy of the system may likewise be written as a function of the other extensive parameters as
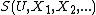 . Suppose that X is one of the
. Suppose that X is one of the 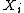 which varies as a system approaches equilibrium, and that it is the only such parameter which is varying. The principle of maximum entropy may then be stated as:
which varies as a system approaches equilibrium, and that it is the only such parameter which is varying. The principle of maximum entropy may then be stated as: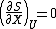 and
and 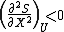 at equilibrium.
at equilibrium.The first condition states that entropy is at an extremum, and the second condition states that entropy is at a maximum. Note that for the partial derivatives, all extensive parameters are assumed constant except for the variables contained in the partial derivative, but only U, S, or X are shown. It follows from the properties of an exact differential (see equation 7 in the exact differential
Exact differential
A mathematical differential is said to be exact, as contrasted with an inexact differential, if it is of the form dQ, for some differentiable function Q....
article) and from the energy/entropy equation of state that, for a closed system:
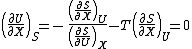
It is seen that the energy is at an extremum at equilibrium. By similar but somewhat more lengthy argument it can be shown that
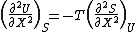
which is greater than zero, showing that the energy is, in fact, at a minimum. (See Callen (1985) chapter 5).
An example
Suppose we have a cylinder containing an ideal gas, with cross sectional area A and a variable height x. Suppose that a weight of mass m has been placed on top of the cylinder. It presses down on the top of the cylinder with a force of mg where g is the acceleration due to gravity.Suppose that x is smaller than its equilibrium value. The upward force of the gas is greater than the downward force of the weight, and if allowed to freely move, the gas in the cylinder would push the weight upward rapidly, and there would be frictional forces that would convert the energy to heat. If we specify that an external agent presses down on the weight so as to very slowly (reversibly) allow the weight to move upward to its equilibrium position, then there will be no heat generated and the entropy of the system will remain constant while energy is transferred as work to the external agent. The total energy of the system at any value of x is given by the internal energy of the gas plus the potential energy of the weight:
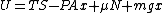
where T is temperature, S is entropy, P is pressure, μ is the chemical potential, N is the number of particles in the gas, and the volume has been written as V=Ax. Since the system is closed, the particle number N is constant and a small change in the energy of the system would be given by:
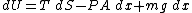
Since the entropy is constant, we may say that dS=0 at equilibrium and by the principle of minimum energy, we may say that dU=0 at equilibrium, yielding the equilibrium condition:
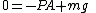
which simply states that the upward gas pressure force (PA) on the upper face of the cylinder is equal to the downward force of the mass due to gravitation (mg).
Thermodynamic potentials
The principle of minimum energy can be generalized to apply to constraints other than fixed entropy. For other constraints, other state functions with dimensions of energy will be minimized. These state functions are known as thermodynamic potentials. Thermodynamic potentials are at first glance just simple algebraic combinations of the energy terms in the expression for the internal energy. For a simple, multicomponent system, the internal energy may be written: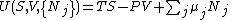
where the intensive parameters (T, P, μj) are functions of the internal energy's natural variables
 via the equations of state. As an example of another thermodynamic potential, the Helmholtz free energy
via the equations of state. As an example of another thermodynamic potential, the Helmholtz free energyHelmholtz free energy
In thermodynamics, the Helmholtz free energy is a thermodynamic potential that measures the “useful” work obtainable from a closed thermodynamic system at a constant temperature and volume...
is written:
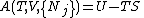
where temperature has replaced entropy as a natural variable. In order to understand the value of the thermodynamic potentials, it is necessary to view them in a different light. They may in fact be seen as (negative) Legendre transforms of the internal energy, in which certain of the extensive parameters are replaced by the derivative of internal energy with respect to that variable (i.e. the conjugate
Conjugate variables (thermodynamics)
In thermodynamics, the internal energy of a system is expressed in terms of pairs of conjugate variables such as temperature/entropy or pressure/volume. In fact all thermodynamic potentials are expressed in terms of conjugate pairs....
to that variable). For example, the Helmholtz free energy may be written:
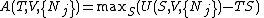
and the maximum will occur when the variable T becomes equal to the temperature since
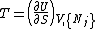
The Helmholtz free energy is a useful quantity when studying thermodynamic transformations in which the temperature is held constant. Although the reduction in the number of variables is a useful simplification, the main advantage comes from the fact that the Helmholtz free energy is minimized at equilibrium with respect to any unconstrained internal variables for a closed system
Closed system
-In physics:In thermodynamics, a closed system can exchange energy , but not matter, with its surroundings.In contrast, an isolated system cannot exchange any of heat, work, or matter with the surroundings, while an open system can exchange all of heat, work and matter.For a simple system, with...
at constant temperature and volume. This follows directly from the principle of minimum energy which states that at constant entropy, the internal energy is minimized. This can be stated as:
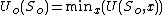
where
 and
and 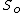 are the value of the internal energy and the (fixed) entropy at equilibrium. The volume and particle number variables have been replaced by x which stands for any internal unconstrained variables.
are the value of the internal energy and the (fixed) entropy at equilibrium. The volume and particle number variables have been replaced by x which stands for any internal unconstrained variables.The minimization is with respect to the unconstrained variables. In the case of chemical reactions this is usually the number of particles or mole fractions, subject to the conservation of elements. At equilibrium, these will take on their equilibrium values, and the internal energy
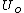 will be a function only of the chosen value of entropy
will be a function only of the chosen value of entropy 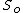 . By the definition of the Legendre transform, the Helmholtz free energy will be:
. By the definition of the Legendre transform, the Helmholtz free energy will be: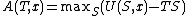
The Helmholtz free energy at equilibrium will be:
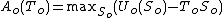
where
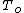 is the (unknown) temperature at equilibrium. Substituting the expression for
is the (unknown) temperature at equilibrium. Substituting the expression for 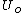 :
: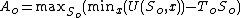
Assuming the order of the extrema can be exchanged:
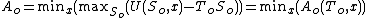
showing that the Helmholtz free energy is minimized at equilibrium.
The Enthalpy
Enthalpy
Enthalpy is a measure of the total energy of a thermodynamic system. It includes the internal energy, which is the energy required to create a system, and the amount of energy required to make room for it by displacing its environment and establishing its volume and pressure.Enthalpy is a...
and Gibbs free energy
Gibbs free energy
In thermodynamics, the Gibbs free energy is a thermodynamic potential that measures the "useful" or process-initiating work obtainable from a thermodynamic system at a constant temperature and pressure...
, are similarly derived.

