
Potassium nonahydridorhenate
Encyclopedia
Potassium nonahydridorhenate is an inorganic compound
with the formula
K2ReH9. This colourless salt
features the ReH92− anion, a rare example of a coordination complex bearing only hydride
ligands.
The study of rhenium
hydrides can be traced to the 1950s and included reports of the "rhenide" anion, supposedly Re−. These reports led to a series of investigations by A. P. Ginsberg and coworkers on the products from the reduction of perrhenate
.
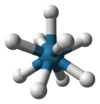 ReH92− is an unusual example of a nine-coordinated complex, the high coordination number being attributed to the small size of the hydride ligand and the high positive charge on the Re(VII) center. The structure consists of a tricapped trigonal prism. The diamagnetic sodium salt, like the analogous technetium
ReH92− is an unusual example of a nine-coordinated complex, the high coordination number being attributed to the small size of the hydride ligand and the high positive charge on the Re(VII) center. The structure consists of a tricapped trigonal prism. The diamagnetic sodium salt, like the analogous technetium
compound, is prepared by treating an ethanol solution of sodium perrhenate
, NaReO4, with sodium metal. Via cation exchange, it can be converted to the corresponding tetraethylammonium
salt, (NEt4)2ReH9.
Inorganic compound
Inorganic compounds have traditionally been considered to be of inanimate, non-biological origin. In contrast, organic compounds have an explicit biological origin. However, over the past century, the classification of inorganic vs organic compounds has become less important to scientists,...
with the formula
Chemical formula
A chemical formula or molecular formula is a way of expressing information about the atoms that constitute a particular chemical compound....
K2ReH9. This colourless salt
Salt
In chemistry, salts are ionic compounds that result from the neutralization reaction of an acid and a base. They are composed of cations and anions so that the product is electrically neutral...
features the ReH92− anion, a rare example of a coordination complex bearing only hydride
Hydride
In chemistry, a hydride is the anion of hydrogen, H−, or, more commonly, a compound in which one or more hydrogen centres have nucleophilic, reducing, or basic properties. In compounds that are regarded as hydrides, hydrogen is bonded to a more electropositive element or group...
ligands.
The study of rhenium
Rhenium
Rhenium is a chemical element with the symbol Re and atomic number 75. It is a silvery-white, heavy, third-row transition metal in group 7 of the periodic table. With an average concentration of 1 part per billion , rhenium is one of the rarest elements in the Earth's crust. The free element has...
hydrides can be traced to the 1950s and included reports of the "rhenide" anion, supposedly Re−. These reports led to a series of investigations by A. P. Ginsberg and coworkers on the products from the reduction of perrhenate
Perrhenate
The perrhenate ion is ReO4− and a perrhenate is a compound containing this ion. Salts of perrhenic acid are known as perrhenates. The perrhenate anion is tetrahedral, as shown by IR and Raman spectroscopy...
.
Structure, synthesis, and properties

Technetium
Technetium is the chemical element with atomic number 43 and symbol Tc. It is the lowest atomic number element without any stable isotopes; every form of it is radioactive. Nearly all technetium is produced synthetically and only minute amounts are found in nature...
compound, is prepared by treating an ethanol solution of sodium perrhenate
Sodium perrhenate
Sodium perrhenate is a white crystalline solid. It is a commonly available precursor for rhenium compounds....
, NaReO4, with sodium metal. Via cation exchange, it can be converted to the corresponding tetraethylammonium
Tetraethylammonium
Tetraethylammonium is a quaternary ammonium cation consisting of four ethyl groups attached to a central nitrogen atom. Like other members of its class, it can be used to alter a compound's solubility by displacing hard acids with this comparatively softer acid...
salt, (NEt4)2ReH9.

