Perrhenate
Encyclopedia
The perrhenate ion is ReO4− and a perrhenate is a compound containing this ion. Salts of perrhenic acid
are known as perrhenates. The perrhenate anion is tetrahedral, as shown by IR and Raman spectroscopy. As in the parent acid rhenium has an oxidation state
of +7 with a d0 configuration.[4]
The perrhenate anion is stable over a broad pH range and can be precipitated from solutions with the use of organic cations. At high pH meso ReO53− forms.
The perrhenate anion is also used as a weakly coordinating anion. It is a weaker base than Cl− or Br− but stronger than ClO4− or BF4− .[4] Solid perrhenate salts takes on the color of the cation. Of the alkali metals salts, KReO4 is common and is the basis of the recovery of rhenium compounds from natural ores and laboratory residues.[6] Structurally the behavior of ReO4− resembles that of perchlorate
, ClO4− : perrhenate and perchlorate salts are often isomorphous.
Typical perrhenate salt formation using alkali earth metals. HReO4 + KCl (solid) → KReO4 + HCl [4]
Silver and other metals can also be used:[5]
AgReO4 + (CH3)3SiCl → (CH3)3SiOReO3 + AgCl
The perrhenate ion will also react with the cyanide
ion. ReO4− + KCN + N2H5OH → K3[ReO2(CN)4]
Different sulfur containing reactions include the formation of thio-perrhenates with the perrhenate ion ReO4− + H2S → ReO3S− + H2O. [7]
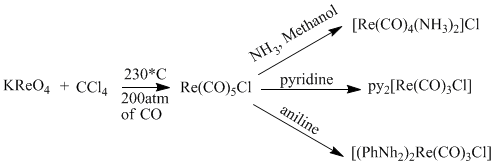
Using one of the most common salts, potassium perrhenate to produce a very useful rhenium compounds that is very stable and allows for numerous further reactions.[6]
Perrhenate is analogous to permanganate
but it has little oxidizing power.
Perrhenic acid
Perrhenic acid is the chemical compound with the formula Re2O72. It is obtained by evaporating aqueous solutions of Re2O7. Conventionally, perrhenic acid is considered to have the formula HReO4, and a species of this formula forms when rhenium oxide sublimes in the presence of water or steam...
are known as perrhenates. The perrhenate anion is tetrahedral, as shown by IR and Raman spectroscopy. As in the parent acid rhenium has an oxidation state
Oxidation state
In chemistry, the oxidation state is an indicator of the degree of oxidation of an atom in a chemical compound. The formal oxidation state is the hypothetical charge that an atom would have if all bonds to atoms of different elements were 100% ionic. Oxidation states are typically represented by...
of +7 with a d0 configuration.[4]
The perrhenate anion is stable over a broad pH range and can be precipitated from solutions with the use of organic cations. At high pH meso ReO53
The perrhenate anion is also used as a weakly coordinating anion. It is a weaker base than Cl
Perchlorate
Perchlorates are the salts derived from perchloric acid . They occur both naturally and through manufacturing. They have been used as a medicine for more than 50 years to treat thyroid gland disorders. They are used extensively within the pyrotechnics industry, and ammonium perchlorate is also a...
, ClO4
Typical perrhenate salt formation using alkali earth metals. HReO4 + KCl (solid) → KReO4 + HCl [4]
Silver and other metals can also be used:[5]
AgReO4 + (CH3)3SiCl → (CH3)3SiOReO3 + AgCl
The perrhenate ion will also react with the cyanide
Cyanide
A cyanide is a chemical compound that contains the cyano group, -C≡N, which consists of a carbon atom triple-bonded to a nitrogen atom. Cyanides most commonly refer to salts of the anion CN−. Most cyanides are highly toxic....
ion. ReO4
Different sulfur containing reactions include the formation of thio-perrhenates with the perrhenate ion ReO4
Using one of the most common salts, potassium perrhenate to produce a very useful rhenium compounds that is very stable and allows for numerous further reactions.[6]

Perrhenate is analogous to permanganate
Permanganate
A permanganate is the general name for a chemical compound containing the manganate ion, . Because manganese is in the +7 oxidation state, the permanganate ion is a strong oxidizing agent. The ion has tetrahedral geometry...
but it has little oxidizing power.

