
Parent hydride
Encyclopedia
In IUPAC nomenclature
, a parent hydride is an unbranched acyclic or cyclic structure to which only hydrogen atoms are attached.
Parent hydrides are parent structure
s that contain one or more hydrogen atoms. They are the basic structures used in substitutive nomenclature.
The bonding number of a skeletal atom in a parent hydride is the sum of the total number of valence bonds to adjacent skeletal atoms, if any, and the number of attached hydrogen atoms. In SH2, for example, S has bonding number 2.
, thus with only hydrogens attached to it. They have a defined standard population of hydrogen atoms. Acyclic parent hydrides are always saturated and unbranched. Cyclic parent hydrides are usually either fully saturated or fully unsaturated (containing the maximum number of double bonds). Some combinations of rings or combinations of cyclic and acyclic hydride
s may be partially saturated.
All elements have 'standard bonding numbers', that is for nitrogen
and phosphorus
3, for carbon
and silicon
4, etcetera.
The names of parent hydrides are ending with 'ane', analogous with the nomenclature for alkanes. Unsaturated hydrides are given the ending 'ene' or 'yne', for example, 'diene' for two double bonds. For non-carbon homocyclic compounds with 3 to 10 membered rings the Hantzsch–Widman nomenclature
is preferred. Derivatives
of parent hydrides get the name of the parent hydride, along with prefixes or suffixes appropriate to the substituent
s that replace the hydrogen atoms.
Parent hydrides are not only used in organic chemistry nomenclature
, but also in inorganic chemistry.
BH3 (borane), CH4 (methane; not carbane!), SiH4 (silane), NH3 (azane), PH3 (phosphane), H2S (sulfane) and H2O (oxidane).
IUPAC nomenclature
A chemical nomenclature is a set of rules to generate systematic names for chemical compounds. The nomenclature used most frequently worldwide is the one created and developed by the International Union of Pure and Applied Chemistry ....
, a parent hydride is an unbranched acyclic or cyclic structure to which only hydrogen atoms are attached.
Parent hydrides are parent structure
Parent structure
In IUPAC nomenclature, a parent structure, parent compound, parent name or simply parent is the denotation for a compound consisting of an unbranched chain of skeletal atoms , or consisting of an unsubstituted monocyclic or polycyclic ring system.Parent structures bearing one or more functional...
s that contain one or more hydrogen atoms. They are the basic structures used in substitutive nomenclature.
The bonding number of a skeletal atom in a parent hydride is the sum of the total number of valence bonds to adjacent skeletal atoms, if any, and the number of attached hydrogen atoms. In SH2, for example, S has bonding number 2.
Parent hydrides in substitutive nomenclature
Parent hydrides are compounds with an unsubstituted skeletonSkeletal formula
The skeletal formula of an organic compound is a shorthand representation of its molecular structure, developed by the organic chemist, Friedrich August Kekulé von Stradonitz. Skeletal formulae are ubiquitous in organic chemistry, because they are relatively quick and simple to draw. Carbon and...
, thus with only hydrogens attached to it. They have a defined standard population of hydrogen atoms. Acyclic parent hydrides are always saturated and unbranched. Cyclic parent hydrides are usually either fully saturated or fully unsaturated (containing the maximum number of double bonds). Some combinations of rings or combinations of cyclic and acyclic hydride
Hydride
In chemistry, a hydride is the anion of hydrogen, H−, or, more commonly, a compound in which one or more hydrogen centres have nucleophilic, reducing, or basic properties. In compounds that are regarded as hydrides, hydrogen is bonded to a more electropositive element or group...
s may be partially saturated.
All elements have 'standard bonding numbers', that is for nitrogen
Nitrogen
Nitrogen is a chemical element that has the symbol N, atomic number of 7 and atomic mass 14.00674 u. Elemental nitrogen is a colorless, odorless, tasteless, and mostly inert diatomic gas at standard conditions, constituting 78.08% by volume of Earth's atmosphere...
and phosphorus
Phosphorus
Phosphorus is the chemical element that has the symbol P and atomic number 15. A multivalent nonmetal of the nitrogen group, phosphorus as a mineral is almost always present in its maximally oxidized state, as inorganic phosphate rocks...
3, for carbon
Carbon
Carbon is the chemical element with symbol C and atomic number 6. As a member of group 14 on the periodic table, it is nonmetallic and tetravalent—making four electrons available to form covalent chemical bonds...
and silicon
Silicon
Silicon is a chemical element with the symbol Si and atomic number 14. A tetravalent metalloid, it is less reactive than its chemical analog carbon, the nonmetal directly above it in the periodic table, but more reactive than germanium, the metalloid directly below it in the table...
4, etcetera.
The names of parent hydrides are ending with 'ane', analogous with the nomenclature for alkanes. Unsaturated hydrides are given the ending 'ene' or 'yne', for example, 'diene' for two double bonds. For non-carbon homocyclic compounds with 3 to 10 membered rings the Hantzsch–Widman nomenclature
Hantzsch–Widman nomenclature
Hantzsch–Widman nomenclature, also called the extended Hantzsch–Widman system, is a type of systematic chemical nomenclature used for naming heterocyclic parent hydrides having no more than ten ring members....
is preferred. Derivatives
Derivative (chemistry)
In chemistry, a derivative is a compound that is derived from a similar compound by some chemical or physical process. In the past it was also used to mean a compound that can be imagined to arise from another compound, if one atom is replaced with another atom or group of atoms, but modern...
of parent hydrides get the name of the parent hydride, along with prefixes or suffixes appropriate to the substituent
Substituent
In organic chemistry and biochemistry, a substituent is an atom or group of atoms substituted in place of a hydrogen atom on the parent chain of a hydrocarbon...
s that replace the hydrogen atoms.
Parent hydrides are not only used in organic chemistry nomenclature
IUPAC nomenclature of organic chemistry
The IUPAC nomenclature of organic chemistry is a systematic method of naming organic chemical compounds as recommended by the International Union of Pure and Applied Chemistry . Ideally, every possible organic compound should have a name from which an unambiguous structural formula can be drawn. ...
, but also in inorganic chemistry.
Examples
Examples of mononuclear parent hydrides (with a single skeletal atom) are:BH3 (borane), CH4 (methane; not carbane!), SiH4 (silane), NH3 (azane), PH3 (phosphane), H2S (sulfane) and H2O (oxidane).
| Some inorganic parent hydrides | |||
|---|---|---|---|
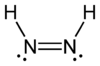 |
|||

