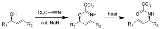
Overman rearrangement
Encyclopedia
The Overman rearrangement is a chemical reaction
that can be described as a Claisen rearrangement
of allylic alcohols to give allylic trichloroacetamides through an imidate intermediate. The Overman rearrangement was discovered in 1974 by Larry Overman.
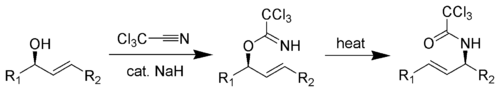 The [3,3]-sigmatropic rearrangement is diastereoselective and can be catalyzed by heat, Hg(II)
The [3,3]-sigmatropic rearrangement is diastereoselective and can be catalyzed by heat, Hg(II)
, or Pd(II)
. The resulting allylamine structures can be transformed into many chemically and biologically important natural and un-natural amino acids (like (1-adamantyl
)glycine).
The Overman rearrangement may also be used for asymmetric synthesis.
Chemical reaction
A chemical reaction is a process that leads to the transformation of one set of chemical substances to another. Chemical reactions can be either spontaneous, requiring no input of energy, or non-spontaneous, typically following the input of some type of energy, such as heat, light or electricity...
that can be described as a Claisen rearrangement
Claisen rearrangement
The Claisen rearrangement is a powerful carbon-carbon bond-forming chemical reaction discovered by Rainer Ludwig Claisen...
of allylic alcohols to give allylic trichloroacetamides through an imidate intermediate. The Overman rearrangement was discovered in 1974 by Larry Overman.

Mercury (element)
Mercury is a chemical element with the symbol Hg and atomic number 80. It is also known as quicksilver or hydrargyrum...
, or Pd(II)
Palladium
Palladium is a chemical element with the chemical symbol Pd and an atomic number of 46. It is a rare and lustrous silvery-white metal discovered in 1803 by William Hyde Wollaston. He named it after the asteroid Pallas, which was itself named after the epithet of the Greek goddess Athena, acquired...
. The resulting allylamine structures can be transformed into many chemically and biologically important natural and un-natural amino acids (like (1-adamantyl
Adamantane
Adamantane is a colorless, crystalline chemical compound with a camphor-like odor. With a formula C10H16, it is a cycloalkane and also the simplest diamondoid. Adamantane molecules consist of three cyclohexane rings arranged in the "armchair" configuration. It is unique in that it is both rigid...
)glycine).
The Overman rearrangement may also be used for asymmetric synthesis.
Further reading
- Isobe, M. et al. Tetrahedron Lett. 1990, 31, 3327.
- Allmendinger, T. et al. Tetrahedron LettersTetrahedron Letters-See also:*Tetrahedron*Tetrahedron: Asymmetry...
1990, 31, 7301. - Nishikawa, T.; Asai, M.; Ohyabu, N.; Isobe, M.; J. Org. Chem. 1998, 63(1), 188-192. (PMID: 11674062)

