
Nuclear density
Encyclopedia
Nuclear density is the density
of the nucleus
of an atom
, averaging about 4×1017 kg/m³. The descriptive term nuclear density is also applied to situations where similarly high densities occur, such as within neutron stars.
The nuclear density in a typical nucleus can be approximately calculated from the size of the nucleus. The radius of a typical nucleus is

where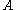 is the mass number and
is the mass number and  is 1.25 fm, with deviations of 0.2 fm from this value. The nucleon
is 1.25 fm, with deviations of 0.2 fm from this value. The nucleon
's density n satisfies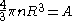
Therefore .
.
Experimentally determined value is .
.
The mass density given above is the product of this by the nucleon
mass 1.67×10−27 kg.
The components of an atom and of an atomic nucleus have varying densities. The proton
is not a fundamental particle, being composed of quark-gluon matter. Its size is approximately 10−15 meters and its density 1018 kg/m³. Using deep inelastic scattering
, it has been estimated that the "size" of an electron
, if it is not a point particle, must be less than 10−17 meters. This would correspond to a density of roughly 1021 kg/m³.
Probing deeper within particles, one finds quarks which appear to be very dense and very hard. There are possibilities for still higher densities when it comes to quark matter, gluon matter, or neutrino matter. In the immediate future, the highest experimentally measurable densities will likely be limited to lepton
s and quark
s.
Density
The mass density or density of a material is defined as its mass per unit volume. The symbol most often used for density is ρ . In some cases , density is also defined as its weight per unit volume; although, this quantity is more properly called specific weight...
of the nucleus
Atomic nucleus
The nucleus is the very dense region consisting of protons and neutrons at the center of an atom. It was discovered in 1911, as a result of Ernest Rutherford's interpretation of the famous 1909 Rutherford experiment performed by Hans Geiger and Ernest Marsden, under the direction of Rutherford. The...
of an atom
Atom
The atom is a basic unit of matter that consists of a dense central nucleus surrounded by a cloud of negatively charged electrons. The atomic nucleus contains a mix of positively charged protons and electrically neutral neutrons...
, averaging about 4×1017 kg/m³. The descriptive term nuclear density is also applied to situations where similarly high densities occur, such as within neutron stars.
The nuclear density in a typical nucleus can be approximately calculated from the size of the nucleus. The radius of a typical nucleus is

where
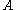 is the mass number and
is the mass number and  is 1.25 fm, with deviations of 0.2 fm from this value. The nucleon
is 1.25 fm, with deviations of 0.2 fm from this value. The nucleonNucleon
In physics, a nucleon is a collective name for two particles: the neutron and the proton. These are the two constituents of the atomic nucleus. Until the 1960s, the nucleons were thought to be elementary particles...
's density n satisfies
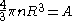
Therefore
 .
.Experimentally determined value is
 .
.The mass density given above is the product of this by the nucleon
Nucleon
In physics, a nucleon is a collective name for two particles: the neutron and the proton. These are the two constituents of the atomic nucleus. Until the 1960s, the nucleons were thought to be elementary particles...
mass 1.67×10−27 kg.
The components of an atom and of an atomic nucleus have varying densities. The proton
Proton
The proton is a subatomic particle with the symbol or and a positive electric charge of 1 elementary charge. One or more protons are present in the nucleus of each atom, along with neutrons. The number of protons in each atom is its atomic number....
is not a fundamental particle, being composed of quark-gluon matter. Its size is approximately 10−15 meters and its density 1018 kg/m³. Using deep inelastic scattering
Deep Inelastic Scattering
Deep inelastic scattering is the name given to a process used to probe the insides of hadrons , using electrons, muons and neutrinos. It provided the first convincing evidence of the reality of quarks, which up until that point had been considered by many to be a purely mathematical phenomenon...
, it has been estimated that the "size" of an electron
Electron
The electron is a subatomic particle with a negative elementary electric charge. It has no known components or substructure; in other words, it is generally thought to be an elementary particle. An electron has a mass that is approximately 1/1836 that of the proton...
, if it is not a point particle, must be less than 10−17 meters. This would correspond to a density of roughly 1021 kg/m³.
Probing deeper within particles, one finds quarks which appear to be very dense and very hard. There are possibilities for still higher densities when it comes to quark matter, gluon matter, or neutrino matter. In the immediate future, the highest experimentally measurable densities will likely be limited to lepton
Lepton
A lepton is an elementary particle and a fundamental constituent of matter. The best known of all leptons is the electron which governs nearly all of chemistry as it is found in atoms and is directly tied to all chemical properties. Two main classes of leptons exist: charged leptons , and neutral...
s and quark
Quark
A quark is an elementary particle and a fundamental constituent of matter. Quarks combine to form composite particles called hadrons, the most stable of which are protons and neutrons, the components of atomic nuclei. Due to a phenomenon known as color confinement, quarks are never directly...
s.
See also
- Nuclear equation of state
- Nuclear matterNuclear matterNuclear matter is an idealized system of interacting nucleons that exists in several phases that as yet are not fully established...
- Quark-gluon plasmaQuark-gluon plasmaA quark–gluon plasma or quark soup is a phase of quantum chromodynamics which exists at extremely high temperature and/or density. This phase consists of asymptotically free quarks and gluons, which are several of the basic building blocks of matter...
- Nuclear compressibility

