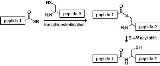
Native chemical ligation
Encyclopedia
Native chemical ligation or NCL is the most widely used form of chemical ligation
, a technique for constructing a large polypeptide from two or more unprotected peptides. In native chemical ligation a peptide containing a C-terminal thioester
reacts with another peptide containing an N-terminal cysteine
residue, in the presence of an added thiol catalyst. In a freely reversible first step, a transthioesterification occurs to yield a thioester-linked intermediate; this intermediate rearranges irreversibly under the usual reaction conditions to form a native amide ('peptide') bond at the ligation site. Native chemical ligation of unprotected peptide segments was developed in the laboratory of Stephen Kent
at The Scripps Research Institute
in 1994. [The transthioesterification/amide-forming intramolecular rearrangement reaction had been reported in 1953 by Theodor Wieland, who was studying possible chemistries for aminoacid addition to a polypeptide chain in protein biosynthesis; Wieland's work led to the 'active ester' method for making protected peptide segments in conventional solution synthesis in organic solvents.]
Native chemical ligation is carried out in aqueous solution and frequently gives near-quantitative yields of the desired ligation product. The challenge is the preparation of the necessary unprotected peptide-thioester building block. Peptide-thioesters are usually prepared by Boc chemistry SPPS; a thioester-containing peptide cannot be synthesized using a nucleophilic base, thus disfavoring Fmoc chemistry. Fmoc chemistry solid phase peptide synthesis techniques for generating peptide-thioesters are known; they make use of modifications of the Kenner 'safety catch' linker. In making peptide segments for use in native chemical ligation, protecting groups that release aldehyde
s or ketones should be avoided since these may cap the N-terminal cysteine. For the same reason, the use of acetone
should be avoided, particularly prior to lyophilization and in washing glassware.
A feature of the native chemical ligation technique is that the product polypeptide chain contains cysteine at the site of ligation. For some proteins, homocysteine
can be used and methylated after ligation to form methionine
, although side reactions can occur in this alkylation step. The cysteine at the ligation site can also be desulfurized to alanine
; more recently, other beta-thiol containing aminoacids have been used for native chemical ligation, followed by desulfurization. Alternatively, thiol-containing ligation auxiliaries can be used that mimic an N-terminal cysteine for the ligation reaction, but which can be removed after synthesis. The use of thiol-containing auxiliaries is not as effective as ligation at a Cys residue.
The payoff in the native chemical ligation method is that coupling long peptides by this technique is in many cases nearly quantitative and provides synthetic access to large peptides and proteins otherwise impossible to make, due to length or decoration by post-translational modification. Native chemical ligation forms the basis of modern chemical protein synthesis, and has been used to prepare numerous proteins and enzymes by total chemical synthesis.
Polypeptide C-terminal thioesters produced by recombinant DNA techniques can be reacted with an N-terminal Cys containing polypeptide by the same native ligation chemistry to provide very large semi-synthetic proteins. Native chemical ligation of this kind using a recombinant polypeptide segment is known as Expressed Protein Ligation. Similarly, a recombinant protein containing an N-terminal Cys can be reacted with a synthetic polypeptide thioester. Thus, native chemical ligation can be used to introduce chemically synthesized segments into recombinant proteins, regardless of size.
and the 203 amino acid HIV-1 protease.
Chemical ligation
Chemical ligation is a set of techniques used for creating long peptide or protein chains. It is the second step of a convergent approach. First, smaller peptides containing 30-50 amino acids are prepared by conventional chemical peptide synthesis. Then, they are completely deprotected...
, a technique for constructing a large polypeptide from two or more unprotected peptides. In native chemical ligation a peptide containing a C-terminal thioester
Thioester
Thioesters are compounds with the functional group C-S-CO-C. They are the product of esterification between a carboxylic acid and a thiol. Thioesters are widespread in biochemistry, the best-known derivative being acetyl-CoA.-Synthesis:...
reacts with another peptide containing an N-terminal cysteine
Cysteine
Cysteine is an α-amino acid with the chemical formula HO2CCHCH2SH. It is a non-essential amino acid, which means that it is biosynthesized in humans. Its codons are UGU and UGC. The side chain on cysteine is thiol, which is polar and thus cysteine is usually classified as a hydrophilic amino acid...
residue, in the presence of an added thiol catalyst. In a freely reversible first step, a transthioesterification occurs to yield a thioester-linked intermediate; this intermediate rearranges irreversibly under the usual reaction conditions to form a native amide ('peptide') bond at the ligation site. Native chemical ligation of unprotected peptide segments was developed in the laboratory of Stephen Kent
Stephen Kent (chemist)
Stephen B. H. Kent is a chemist at the University of Chicago who developed native chemical ligation and also demonstrated the principle that mirror-image amino acids put together to form a protein create a mirror-image protein which, if an enzyme, can catalyze the mirror-image reaction.-External...
at The Scripps Research Institute
The Scripps Research Institute
The Scripps Research Institute is an American medical research facility that focuses on research in the basic biomedical sciences. Headquartered in La Jolla, California, with a sister facility in Jupiter, Florida, the institute is home to 3,000 scientists, technicians, graduate students, and...
in 1994. [The transthioesterification/amide-forming intramolecular rearrangement reaction had been reported in 1953 by Theodor Wieland, who was studying possible chemistries for aminoacid addition to a polypeptide chain in protein biosynthesis; Wieland's work led to the 'active ester' method for making protected peptide segments in conventional solution synthesis in organic solvents.]
Mechanism
NCL involves the coupling of two unprotected peptides, one bearing a C-terminal α-thioester (N-terminal fragment 1) and the other an N-terminal cysteine residue (C-terminal fragment 2). The reaction is initiated by dissolving both peptides in an aqueous buffer at pH 7.0. The mechanism starts with a reversible transthioesterification reaction involving a nucleophilic attack of the N-terminal thiol group of peptide 2 at the α-thioester of peptide 1. The formed intermediate 3 undergoes a spontaneous rearrangement giving a natural peptide bond at the ligation site. The N-terminal cysteine as well as the internal cysteines are able to form the thioester, but only the nucleophilic attack of the primary amine at the N-terminus gives the amide bond.Native chemical ligation is carried out in aqueous solution and frequently gives near-quantitative yields of the desired ligation product. The challenge is the preparation of the necessary unprotected peptide-thioester building block. Peptide-thioesters are usually prepared by Boc chemistry SPPS; a thioester-containing peptide cannot be synthesized using a nucleophilic base, thus disfavoring Fmoc chemistry. Fmoc chemistry solid phase peptide synthesis techniques for generating peptide-thioesters are known; they make use of modifications of the Kenner 'safety catch' linker. In making peptide segments for use in native chemical ligation, protecting groups that release aldehyde
Aldehyde
An aldehyde is an organic compound containing a formyl group. This functional group, with the structure R-CHO, consists of a carbonyl center bonded to hydrogen and an R group....
s or ketones should be avoided since these may cap the N-terminal cysteine. For the same reason, the use of acetone
Acetone
Acetone is the organic compound with the formula 2CO, a colorless, mobile, flammable liquid, the simplest example of the ketones.Acetone is miscible with water and serves as an important solvent in its own right, typically as the solvent of choice for cleaning purposes in the laboratory...
should be avoided, particularly prior to lyophilization and in washing glassware.
A feature of the native chemical ligation technique is that the product polypeptide chain contains cysteine at the site of ligation. For some proteins, homocysteine
Homocysteine
Homocysteine is a non-protein amino acid with the formula HSCH2CH2CHCO2H. It is a homologue of the amino acid cysteine, differing by an additional methylene group. It is biosynthesized from methionine by the removal of its terminal Cε methyl group...
can be used and methylated after ligation to form methionine
Methionine
Methionine is an α-amino acid with the chemical formula HO2CCHCH2CH2SCH3. This essential amino acid is classified as nonpolar. This amino-acid is coded by the codon AUG, also known as the initiation codon, since it indicates mRNA's coding region where translation into protein...
, although side reactions can occur in this alkylation step. The cysteine at the ligation site can also be desulfurized to alanine
Alanine
Alanine is an α-amino acid with the chemical formula CH3CHCOOH. The L-isomer is one of the 20 amino acids encoded by the genetic code. Its codons are GCU, GCC, GCA, and GCG. It is classified as a nonpolar amino acid...
; more recently, other beta-thiol containing aminoacids have been used for native chemical ligation, followed by desulfurization. Alternatively, thiol-containing ligation auxiliaries can be used that mimic an N-terminal cysteine for the ligation reaction, but which can be removed after synthesis. The use of thiol-containing auxiliaries is not as effective as ligation at a Cys residue.
The payoff in the native chemical ligation method is that coupling long peptides by this technique is in many cases nearly quantitative and provides synthetic access to large peptides and proteins otherwise impossible to make, due to length or decoration by post-translational modification. Native chemical ligation forms the basis of modern chemical protein synthesis, and has been used to prepare numerous proteins and enzymes by total chemical synthesis.
Polypeptide C-terminal thioesters produced by recombinant DNA techniques can be reacted with an N-terminal Cys containing polypeptide by the same native ligation chemistry to provide very large semi-synthetic proteins. Native chemical ligation of this kind using a recombinant polypeptide segment is known as Expressed Protein Ligation. Similarly, a recombinant protein containing an N-terminal Cys can be reacted with a synthetic polypeptide thioester. Thus, native chemical ligation can be used to introduce chemically synthesized segments into recombinant proteins, regardless of size.
Size limitation
NCL has been proven to be a convenient method for the chemical synthesis of proteins well over 100 amino acids in length, as demonstrated on the 166-amino-acid polypeptide chain of the synthetic variant of the erythropoiesis proteinErythropoietin
Erythropoietin, or its alternatives erythropoetin or erthropoyetin or EPO, is a glycoprotein hormone that controls erythropoiesis, or red blood cell production...
and the 203 amino acid HIV-1 protease.

