LIESST
Encyclopedia
In chemistry and physics, LIESST (Light-Induced Excited Spin-State Trapping) is a method of changing the electronic spin state of a compound by means of irradiation with light.
 Many transition metal
Many transition metal
complexes with electronic configuration d4-d7 are capable of spin crossover
(and d8 when molecular symmetry is lower than Oh). Spin crossover refers to where a transition from the high spin (HS) state to the low spin (LS) state or vice versa occurs. Alternatives to LIESST include using thermal changes and pressure to induce spin crossover. The metal most commonly exhibiting spin crossover is iron, with the first known example, an iron(III) tris(dithiocarbamato) complex, reported by Cambi et al. in 1931.
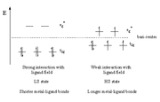
For iron complexes, LIESST involves excitation of the low spin complex with green light to a triplet state. Two successive steps of intersystem crossing
result in the high spin complex. Movement from the high spin complex to the low spin complex requires excitation with red light.

Transition metal
The term transition metal has two possible meanings:*The IUPAC definition states that a transition metal is "an element whose atom has an incomplete d sub-shell, or which can give rise to cations with an incomplete d sub-shell." Group 12 elements are not transition metals in this definition.*Some...
complexes with electronic configuration d4-d7 are capable of spin crossover
Spin crossover
Spin Crossover , sometimes referred to as spin transition or spin equilibrium behavior, is a phenomenon that occurs in some metal complexes wherein the spin state of the complex changes due to external stimuli such as a variation of temperature, pressure, light irradiation or an influence of a...
(and d8 when molecular symmetry is lower than Oh). Spin crossover refers to where a transition from the high spin (HS) state to the low spin (LS) state or vice versa occurs. Alternatives to LIESST include using thermal changes and pressure to induce spin crossover. The metal most commonly exhibiting spin crossover is iron, with the first known example, an iron(III) tris(dithiocarbamato) complex, reported by Cambi et al. in 1931.
For iron complexes, LIESST involves excitation of the low spin complex with green light to a triplet state. Two successive steps of intersystem crossing
Intersystem crossing
Intersystem crossing is a radiationless process involving a transition between two electronic states with different spin multiplicity.-Singlet and triplet states:...
result in the high spin complex. Movement from the high spin complex to the low spin complex requires excitation with red light.

