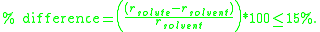Hume-Rothery rules
Encyclopedia
The Hume-Rothery rules, named after William Hume-Rothery
, are a set of basic rules describing the conditions under which an element could dissolve in a metal
, forming a solid solution
. There are two sets of rules, one which refers to substitutional solid solutions, and another which refers to interstitial
solid solutions.
solid solutions, the Hume-Rothery rules are:
William Hume-Rothery
William Hume-Rothery OBE was a British metallurgist who studied the constitution of alloys.- Career :Hume-Rothery was born the son of lawyer Joseph Hume-Rothery in Worcester Park, Surrey but spent his youth in Cheltenham and was educated at Cheltenham College. In 1917 he was made totally deaf by a...
, are a set of basic rules describing the conditions under which an element could dissolve in a metal
Metal
A metal , is an element, compound, or alloy that is a good conductor of both electricity and heat. Metals are usually malleable and shiny, that is they reflect most of incident light...
, forming a solid solution
Solid solution
A solid solution is a solid-state solution of one or more solutes in a solvent. Such a mixture is considered a solution rather than a compound when the crystal structure of the solvent remains unchanged by addition of the solutes, and when the mixture remains in a single homogeneous phase...
. There are two sets of rules, one which refers to substitutional solid solutions, and another which refers to interstitial
Interstitial defect
Interstitials are a variety of crystallographic defects, i.e. atoms which occupy a site in the crystal structure at which there is usually not an atom, or two or more atoms sharing one or more lattice sites such that the number of atoms is larger than the number of lattice sites.They are generally...
solid solutions.
Substitutional Solid Solution Rules
For substitutional solid solutions, the Hume-Rothery rules are:- 1. The atomic radiiAtomic radiusThe atomic radius of a chemical element is a measure of the size of its atoms, usually the mean or typical distance from the nucleus to the boundary of the surrounding cloud of electrons...
of the solute and solventSolventA solvent is a liquid, solid, or gas that dissolves another solid, liquid, or gaseous solute, resulting in a solution that is soluble in a certain volume of solvent at a specified temperature...
atoms must differ by no more than 15%:
- 2. The crystal structureCrystal structureIn mineralogy and crystallography, crystal structure is a unique arrangement of atoms or molecules in a crystalline liquid or solid. A crystal structure is composed of a pattern, a set of atoms arranged in a particular way, and a lattice exhibiting long-range order and symmetry...
s of solute and solvent must match.
- 3. Complete solubilitySolubilitySolubility is the property of a solid, liquid, or gaseous chemical substance called solute to dissolve in a solid, liquid, or gaseous solvent to form a homogeneous solution of the solute in the solvent. The solubility of a substance fundamentally depends on the used solvent as well as on...
occurs when the solvent and solute have the same valencyValence (chemistry)In chemistry, valence, also known as valency or valence number, is a measure of the number of bonds formed by an atom of a given element. "Valence" can be defined as the number of valence bonds...
. A metal will dissolve a metal of higher valency to a greater extent than one of lower valency.
- 4. The solute and solvent should have similar electronegativityElectronegativityElectronegativity, symbol χ , is a chemical property that describes the tendency of an atom or a functional group to attract electrons towards itself. An atom's electronegativity is affected by both its atomic number and the distance that its valence electrons reside from the charged nucleus...
. If the electronegativity difference is too great, the metals will tend to form intermetallic compoundsIntermetallicsIntermetallics or intermetallic compounds is a term that is used in a number of different ways. Most commonly it refers to solid-state phases involving metals. There is a "research definition" adhered to generally in scientific publications, and a wider "common use" term...
instead of solid solutions.
Interstitial Solid Solution Rules
For interstitialInterstitial defect
Interstitials are a variety of crystallographic defects, i.e. atoms which occupy a site in the crystal structure at which there is usually not an atom, or two or more atoms sharing one or more lattice sites such that the number of atoms is larger than the number of lattice sites.They are generally...
solid solutions, the Hume-Rothery rules are:
- 1. Solute atoms must be smaller than the interstitial sites in the solvent lattice.
- 2. The solute and solvent should have similar electronegativityElectronegativityElectronegativity, symbol χ , is a chemical property that describes the tendency of an atom or a functional group to attract electrons towards itself. An atom's electronegativity is affected by both its atomic number and the distance that its valence electrons reside from the charged nucleus...
.