Heteroazeotrope
Encyclopedia
A heteroazeotrope is an azeotrope
where the vapour phase coexists with two liquid phases.
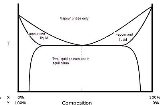
Sketch of a T-x/y equilibrium curve of a typical heteroazeotropic mixture

In this case on the plates can be two liquid phases and the top vapour condensate splits in two liquid phases, which can be separated in a decanter.
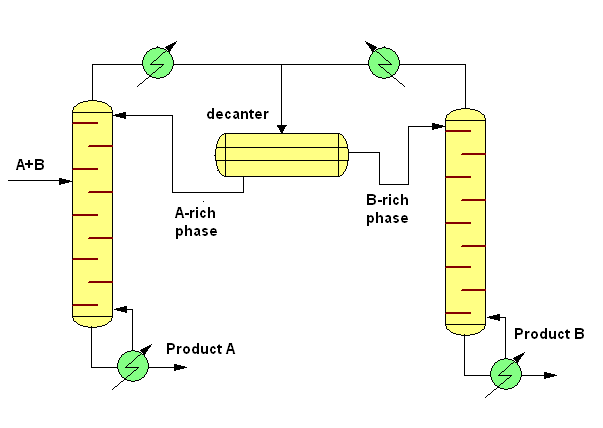
The simplest case of continuous heteroazeotropic distillation is the separation of a binary heterogeneous azeotropic mixture. In this case the system contains two columns and a decanter. The fresh feed (A-B) is added into the first column. (The feed may also be added into the decanter directly or into the second column depending on the composition of the mixture). From the decanter the A-rich phase is withdrawn as reflux into the first column while the B-rich phase is withdrawn as reflux into the second column. This mean the first column produces "A" and the second column produces "B" as a bottoms product. In the industry the butanol-water mixture is separated with this technique.
At the previous case the binary system forms already a heterogeneous azeotrope. The other application of the heteroazeotropic distillation is the separation of a binary system (A-B) forming a homogeneous azeotrope. In this case an entrainer or solvent is added to the mixture in order to form an heteroazeotrope with one or both of the components in order to help the separation of the original A-B mixture.
low relative volatility (low α) mixtures. A third component (entrainer, E) is added to the
binary A-B mixture, which makes the separation of A and B possible. The entrainer forms a
heteroazeotrope with at least one (and preferably with only one (selective entrainer)) of the
original components.
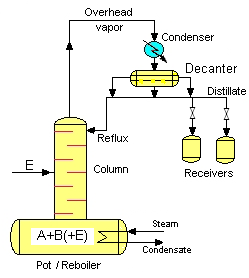
The main parts of the conventional batch distillation columns are the followings:
- pot (include reboiler)
- column
- condenser to condense the top vapour
- product receivers
- (entrianer fed)
In case of the heteroazeotropic distillation the equipment is completed with a decanter, where the two liquid phases are split.
Three different cases are possible for the addition of the entrainer:
1, Batch Addition of the Entrainer: The total quantity of the entrainer is added to the charge before the start of the procedure.
2, Continuous Entrainer Feeding: The total quantity of the entrainer is introduced continuously to the column.
3, Mixed Addition of the Entrainer: The combination of the batch addition and continuous feeding of the entrainer. We added one part of the entrainer to the charge before the start of the distillation and the other part continuously during distillation.
In the last years the batch heteroazeotropic distillation comes into prominenece so several studies were published. The heteroazeotropic batch distillation was investigated by feasibility studies, rigorous simulation calculations and laboratory experiments. Feasibility analysis is conducted in Modla et al. and Rodriguez-Donis et al. for the separation of low-relative-volatility and azeotropic mixtures by heterogeneous batch distillation in a batch rectifier. Rodriguez-Donis et al. were the first to provide the entrainer selection rules. The feasibility methods was extended and modified by Rodriguez-Donis et al.,, Rodriguez-Donis et al., (2005), Skouras et al., and Lang and Modla. Varga applied these feasibility studies in her thesis. Experimental result was published by Rodriguez-Donis et al., Xu and Wand, Van Kaam and others.
Azeotrope
An azeotrope is a mixture of two or more liquids in such a ratio that its composition cannot be changed by simple distillation. This occurs because, when an azeotrope is boiled, the resulting vapor has the same ratio of constituents as the original mixture....
where the vapour phase coexists with two liquid phases.
Sketch of a T-x/y equilibrium curve of a typical heteroazeotropic mixture

Examples of heteroazeotropes
- BenzeneBenzeneBenzene is an organic chemical compound. It is composed of 6 carbon atoms in a ring, with 1 hydrogen atom attached to each carbon atom, with the molecular formula C6H6....
- WaterWaterWater is a chemical substance with the chemical formula H2O. A water molecule contains one oxygen and two hydrogen atoms connected by covalent bonds. Water is a liquid at ambient conditions, but it often co-exists on Earth with its solid state, ice, and gaseous state . Water also exists in a...
NBP 69.2 °C - DichloromethaneDichloromethaneDichloromethane is an organic compound with the formula CH2Cl2. This colorless, volatile liquid with a moderately sweet aroma is widely used as a solvent. Although it is not miscible with water, it is miscible with many organic solvents...
- Water NBP 38.5 °C - Butanol - Water NBP 93.5 °C
Continuous heteroazeotropic distillation
Heterogeneous distillation means that during the distillation the liquid phase of the mixture is immiscible.In this case on the plates can be two liquid phases and the top vapour condensate splits in two liquid phases, which can be separated in a decanter.
The simplest case of continuous heteroazeotropic distillation is the separation of a binary heterogeneous azeotropic mixture. In this case the system contains two columns and a decanter. The fresh feed (A-B) is added into the first column. (The feed may also be added into the decanter directly or into the second column depending on the composition of the mixture). From the decanter the A-rich phase is withdrawn as reflux into the first column while the B-rich phase is withdrawn as reflux into the second column. This mean the first column produces "A" and the second column produces "B" as a bottoms product. In the industry the butanol-water mixture is separated with this technique.

At the previous case the binary system forms already a heterogeneous azeotrope. The other application of the heteroazeotropic distillation is the separation of a binary system (A-B) forming a homogeneous azeotrope. In this case an entrainer or solvent is added to the mixture in order to form an heteroazeotrope with one or both of the components in order to help the separation of the original A-B mixture.
Batch heteroazeotropic distillation
Batch heteroazeotropic distillation is an efficient method for the separation of azeotropic andlow relative volatility (low α) mixtures. A third component (entrainer, E) is added to the
binary A-B mixture, which makes the separation of A and B possible. The entrainer forms a
heteroazeotrope with at least one (and preferably with only one (selective entrainer)) of the
original components.
The main parts of the conventional batch distillation columns are the followings:
- pot (include reboiler)
- column
- condenser to condense the top vapour
- product receivers
- (entrianer fed)
In case of the heteroazeotropic distillation the equipment is completed with a decanter, where the two liquid phases are split.

Three different cases are possible for the addition of the entrainer:
1, Batch Addition of the Entrainer: The total quantity of the entrainer is added to the charge before the start of the procedure.
2, Continuous Entrainer Feeding: The total quantity of the entrainer is introduced continuously to the column.
3, Mixed Addition of the Entrainer: The combination of the batch addition and continuous feeding of the entrainer. We added one part of the entrainer to the charge before the start of the distillation and the other part continuously during distillation.
In the last years the batch heteroazeotropic distillation comes into prominenece so several studies were published. The heteroazeotropic batch distillation was investigated by feasibility studies, rigorous simulation calculations and laboratory experiments. Feasibility analysis is conducted in Modla et al. and Rodriguez-Donis et al. for the separation of low-relative-volatility and azeotropic mixtures by heterogeneous batch distillation in a batch rectifier. Rodriguez-Donis et al. were the first to provide the entrainer selection rules. The feasibility methods was extended and modified by Rodriguez-Donis et al.,, Rodriguez-Donis et al., (2005), Skouras et al., and Lang and Modla. Varga applied these feasibility studies in her thesis. Experimental result was published by Rodriguez-Donis et al., Xu and Wand, Van Kaam and others.

