
Graham's law
Encyclopedia
Graham's law, known as Graham's law of effusion
, was formulated by Scottish physical chemist Thomas Graham
in 1846. Graham found experimentally that the rate of effusion
of a gas is inversely proportional to the square root of the mass of its particles. This formula can be written as:

where:
Graham's law states that the rate of effusion of a gas is inversely proportional to the square root of its molecular weight. Thus, if the molecular weight of one gas is four times that of another, it would diffuse through a porous plug or escape through a small pinhole in a vessel at half the rate of the other. A complete theoretical explanation of Graham's law was provided years later by the kinetic theory of gases
. Graham's law provides a basis for separating isotopes by diffusion — a method that came to play a crucial role in the development of the atomic bomb.
Graham's law is most accurate for molecular effusion
which involves the movement of one gas at a time through a hole. It is only approximate for diffusion
of one gas in another or in air, as these processes involve the movement of more than one gas.
chemist Johann Döbereiner that hydrogen gas diffused out of a small crack in a glass bottle faster than the surrounding air diffused in to replace it. Graham measured the rate of diffusion of gases through plaster plugs, through very fine tubes, and through small orifices. In this way he slowed down the process so that it could be studied quantitatively. He first stated the law as we know it today in 1831. Graham went on to study the diffusion of substances in solution and in the process made the discovery that some apparent solutions actually are suspensions
of particles too large to pass through a parchment filter. He termed these materials colloid
s, a term that has come to denote an important class of finely divided materials.
At the time Graham did his work the concept of molecular weight was being established, in large part through measurements of gases. Italian physicist Amadeo Avogadro had suggested in 1811 that equal volumes of different gases contain equal numbers of molecules. Thus, the relative molecular weights of two gases are equal to the ratio of weights of equal volumes of the gases. Avogadro's insight together with other studies of gas behaviour provided a basis for later theoretical work by Scottish physicist James Clerk Maxwell
to explain the properties of gases as collections of small particles moving through largely empty space.
Perhaps the greatest success of the kinetic theory of gases, as it came to be called, was the discovery that for gases, the temperature as measured on the Kelvin
(absolute) temperature scale is directly proportional to the average kinetic energy of the gas molecules. The kinetic energy of any object is equal to one-half its mass times the square of its velocity. Thus, to have equal kinetic energies, the velocities of two different molecules would have to be in inverse proportion to the square roots of their masses. The rate of effusion is determined by the number of molecules entering an aperture per unit time, and hence by the average molecular velocity. Graham's law for diffusion could thus be understood as a consequence of the molecular kinetic energies being equal at the same temperature.
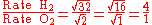
Therefore, hydrogen molecules effuse four times faster than those of oxygen.
Graham's Law can also be used to find the approximate molecular weight of a gas if one gas is a known species, and if there is a specific ratio between the rates of two gases (such as in the previous example). The equation can be solved for either one of the molecular weights provided the subscripts are consistent.
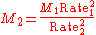
Graham's law was the basis for separating 235U from 238U found in natural uraninite
(uranium ore) during the Manhattan project
to build the first atomic bomb. The United States government built a gaseous diffusion plant at the then phenomenal cost of $100 million in Clinton, Tennessee. In this plant, uranium
from uranium ore was first converted to uranium hexafluoride
and then forced repeatedly to diffuse through porous barriers, each time becoming a little more enriched in the slightly lighter 235U isotope.
Effusion
In physics, effusion is the process in which individual molecules flow through a hole without collisions between molecules. This occurs if the diameter of the hole is considerably smaller than the mean free path of the molecules...
, was formulated by Scottish physical chemist Thomas Graham
Thomas Graham (chemist)
Thomas Graham FRS was a nineteenth-century Scottish chemist who is best-remembered today for his pioneering work in dialysis and the diffusion of gases.- Life and work :...
in 1846. Graham found experimentally that the rate of effusion
Effusion
In physics, effusion is the process in which individual molecules flow through a hole without collisions between molecules. This occurs if the diameter of the hole is considerably smaller than the mean free path of the molecules...
of a gas is inversely proportional to the square root of the mass of its particles. This formula can be written as:

where:
- Rate1 is the rate of effusion of the first gas (volume or number of moles per unit time).
- Rate2 is the rate of effusion for the second gas.
- M1 is the molar massMolar massMolar mass, symbol M, is a physical property of a given substance , namely its mass per amount of substance. The base SI unit for mass is the kilogram and that for amount of substance is the mole. Thus, the derived unit for molar mass is kg/mol...
of gas 1 - M2 is the molar mass of gas 2.
Graham's law states that the rate of effusion of a gas is inversely proportional to the square root of its molecular weight. Thus, if the molecular weight of one gas is four times that of another, it would diffuse through a porous plug or escape through a small pinhole in a vessel at half the rate of the other. A complete theoretical explanation of Graham's law was provided years later by the kinetic theory of gases
Kinetic theory
The kinetic theory of gases describes a gas as a large number of small particles , all of which are in constant, random motion. The rapidly moving particles constantly collide with each other and with the walls of the container...
. Graham's law provides a basis for separating isotopes by diffusion — a method that came to play a crucial role in the development of the atomic bomb.
Graham's law is most accurate for molecular effusion
Effusion
In physics, effusion is the process in which individual molecules flow through a hole without collisions between molecules. This occurs if the diameter of the hole is considerably smaller than the mean free path of the molecules...
which involves the movement of one gas at a time through a hole. It is only approximate for diffusion
Diffusion
Molecular diffusion, often called simply diffusion, is the thermal motion of all particles at temperatures above absolute zero. The rate of this movement is a function of temperature, viscosity of the fluid and the size of the particles...
of one gas in another or in air, as these processes involve the movement of more than one gas.
History
Graham's research on the diffusion of gases was triggered by his reading about the observation of GermanGermany
Germany , officially the Federal Republic of Germany , is a federal parliamentary republic in Europe. The country consists of 16 states while the capital and largest city is Berlin. Germany covers an area of 357,021 km2 and has a largely temperate seasonal climate...
chemist Johann Döbereiner that hydrogen gas diffused out of a small crack in a glass bottle faster than the surrounding air diffused in to replace it. Graham measured the rate of diffusion of gases through plaster plugs, through very fine tubes, and through small orifices. In this way he slowed down the process so that it could be studied quantitatively. He first stated the law as we know it today in 1831. Graham went on to study the diffusion of substances in solution and in the process made the discovery that some apparent solutions actually are suspensions
Suspension (chemistry)
In chemistry, a suspension is a heterogeneous fluid containing solid particles that are sufficiently large for sedimentation. Usually they must be larger than 1 micrometer. The internal phase is dispersed throughout the external phase through mechanical agitation, with the use of certain...
of particles too large to pass through a parchment filter. He termed these materials colloid
Colloid
A colloid is a substance microscopically dispersed evenly throughout another substance.A colloidal system consists of two separate phases: a dispersed phase and a continuous phase . A colloidal system may be solid, liquid, or gaseous.Many familiar substances are colloids, as shown in the chart below...
s, a term that has come to denote an important class of finely divided materials.
At the time Graham did his work the concept of molecular weight was being established, in large part through measurements of gases. Italian physicist Amadeo Avogadro had suggested in 1811 that equal volumes of different gases contain equal numbers of molecules. Thus, the relative molecular weights of two gases are equal to the ratio of weights of equal volumes of the gases. Avogadro's insight together with other studies of gas behaviour provided a basis for later theoretical work by Scottish physicist James Clerk Maxwell
James Clerk Maxwell
James Clerk Maxwell of Glenlair was a Scottish physicist and mathematician. His most prominent achievement was formulating classical electromagnetic theory. This united all previously unrelated observations, experiments and equations of electricity, magnetism and optics into a consistent theory...
to explain the properties of gases as collections of small particles moving through largely empty space.
Perhaps the greatest success of the kinetic theory of gases, as it came to be called, was the discovery that for gases, the temperature as measured on the Kelvin
Kelvin
The kelvin is a unit of measurement for temperature. It is one of the seven base units in the International System of Units and is assigned the unit symbol K. The Kelvin scale is an absolute, thermodynamic temperature scale using as its null point absolute zero, the temperature at which all...
(absolute) temperature scale is directly proportional to the average kinetic energy of the gas molecules. The kinetic energy of any object is equal to one-half its mass times the square of its velocity. Thus, to have equal kinetic energies, the velocities of two different molecules would have to be in inverse proportion to the square roots of their masses. The rate of effusion is determined by the number of molecules entering an aperture per unit time, and hence by the average molecular velocity. Graham's law for diffusion could thus be understood as a consequence of the molecular kinetic energies being equal at the same temperature.
Example
Let gas 1 be H2 and gas 2 be O2.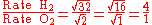
Therefore, hydrogen molecules effuse four times faster than those of oxygen.
Graham's Law can also be used to find the approximate molecular weight of a gas if one gas is a known species, and if there is a specific ratio between the rates of two gases (such as in the previous example). The equation can be solved for either one of the molecular weights provided the subscripts are consistent.
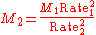
Graham's law was the basis for separating 235U from 238U found in natural uraninite
Uraninite
Uraninite is a radioactive, uranium-rich mineral and ore with a chemical composition that is largely UO2, but also contains UO3 and oxides of lead, thorium, and rare earth elements...
(uranium ore) during the Manhattan project
Manhattan Project
The Manhattan Project was a research and development program, led by the United States with participation from the United Kingdom and Canada, that produced the first atomic bomb during World War II. From 1942 to 1946, the project was under the direction of Major General Leslie Groves of the US Army...
to build the first atomic bomb. The United States government built a gaseous diffusion plant at the then phenomenal cost of $100 million in Clinton, Tennessee. In this plant, uranium
Uranium
Uranium is a silvery-white metallic chemical element in the actinide series of the periodic table, with atomic number 92. It is assigned the chemical symbol U. A uranium atom has 92 protons and 92 electrons, of which 6 are valence electrons...
from uranium ore was first converted to uranium hexafluoride
Uranium hexafluoride
Uranium hexafluoride , referred to as "hex" in the nuclear industry, is a compound used in the uranium enrichment process that produces fuel for nuclear reactors and nuclear weapons. It forms solid grey crystals at standard temperature and pressure , is highly toxic, reacts violently with water...
and then forced repeatedly to diffuse through porous barriers, each time becoming a little more enriched in the slightly lighter 235U isotope.
See also
- Gas lawsGas lawsThe early gas laws were developed at the end of the 18th century, when scientists began to realize that relationships between the pressure, volume and temperature of a sample of gas could be obtained which would hold for all gases...
- Scientific laws named after peopleScientific laws named after peopleThis is a list of scientific laws named after people . For other lists of eponyms, see eponym.-See also:* Eponym* Fields of science...
- ViscosityViscosityViscosity is a measure of the resistance of a fluid which is being deformed by either shear or tensile stress. In everyday terms , viscosity is "thickness" or "internal friction". Thus, water is "thin", having a lower viscosity, while honey is "thick", having a higher viscosity...
- Drag (physics)Drag (physics)In fluid dynamics, drag refers to forces which act on a solid object in the direction of the relative fluid flow velocity...

