
Glycopeptide
Encyclopedia
Glycopeptides are peptide
s that contain carbohydrate
moieties (glycans) covalently attached to the side chains of the amino acid
residues that constitute the peptide. Over the past few decades it has been recognised that glycans on cell surface (attached to membrane proteins or lipids) and those bound to proteins (glycoproteins) play a critical role in biology. For example these constructs have been showed to play important roles in fertilization, the immune system
, brain development, the endocrine system
and inflammation
. The synthesis
of glycopeptides provides biological probes for researchers to elucidate glycan function in nature and products that have useful therapeutic and biotechnological applications.
(Asn, N) residue, and are amongst the most common linkages found in nature5. Although the majority of N-linked glycans take the form GlcNAc-β-Asn other less common structural linkages such as GlcNac-α-Asn and Glc-Asn have been observed.



 Several methods exist for the synthesis of monosaccharide amino acid building block as illustrated below.
Several methods exist for the synthesis of monosaccharide amino acid building block as illustrated below.
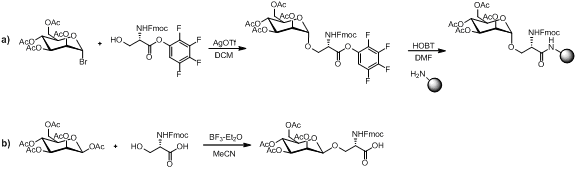 Provided the monosaccharide amino acid building block is stable to peptide coupling conditions, amine deprotection conditions and resin cleavage. Linear assembly remains a popular strategy for the synthesis of glycopeptides with many examples in the literature. In the convergent assembly strategy a peptide chain and glycan residue are first synthesis separately. Then the glycan is glycosylated onto a specific residue of the peptide chain. This approach is not as popular as the linear strategy due to the poor reaction yields in the glycosylation step.
Provided the monosaccharide amino acid building block is stable to peptide coupling conditions, amine deprotection conditions and resin cleavage. Linear assembly remains a popular strategy for the synthesis of glycopeptides with many examples in the literature. In the convergent assembly strategy a peptide chain and glycan residue are first synthesis separately. Then the glycan is glycosylated onto a specific residue of the peptide chain. This approach is not as popular as the linear strategy due to the poor reaction yields in the glycosylation step.
, or NCL, is a convergent synthetic strategy based on the linear coupling of glycopeptide fragments. This technique makes use of the chemoselective reaction between a N-terminal cysteine residue on one peptide fragment with a thio-ester on the C-terminus of the other peptide fragment as illustrated below.
 Unlike standard SPPS (which is limited to 50 amino acid residue) NCL allows the construction of large glycopeptides. However the strategy is limited by the fact that it requires a cysteine residue at N-terminus, an amino acid residue that is rare in nature. However this problem has partly been address by the selective desulfurization of the cysteine residue to an alanine.
Unlike standard SPPS (which is limited to 50 amino acid residue) NCL allows the construction of large glycopeptides. However the strategy is limited by the fact that it requires a cysteine residue at N-terminus, an amino acid residue that is rare in nature. However this problem has partly been address by the selective desulfurization of the cysteine residue to an alanine.
Peptide
Peptides are short polymers of amino acid monomers linked by peptide bonds. They are distinguished from proteins on the basis of size, typically containing less than 50 monomer units. The shortest peptides are dipeptides, consisting of two amino acids joined by a single peptide bond...
s that contain carbohydrate
Carbohydrate
A carbohydrate is an organic compound with the empirical formula ; that is, consists only of carbon, hydrogen, and oxygen, with a hydrogen:oxygen atom ratio of 2:1 . However, there are exceptions to this. One common example would be deoxyribose, a component of DNA, which has the empirical...
moieties (glycans) covalently attached to the side chains of the amino acid
Amino acid
Amino acids are molecules containing an amine group, a carboxylic acid group and a side-chain that varies between different amino acids. The key elements of an amino acid are carbon, hydrogen, oxygen, and nitrogen...
residues that constitute the peptide. Over the past few decades it has been recognised that glycans on cell surface (attached to membrane proteins or lipids) and those bound to proteins (glycoproteins) play a critical role in biology. For example these constructs have been showed to play important roles in fertilization, the immune system
Immune system
An immune system is a system of biological structures and processes within an organism that protects against disease by identifying and killing pathogens and tumor cells. It detects a wide variety of agents, from viruses to parasitic worms, and needs to distinguish them from the organism's own...
, brain development, the endocrine system
Endocrine system
In physiology, the endocrine system is a system of glands, each of which secretes a type of hormone directly into the bloodstream to regulate the body. The endocrine system is in contrast to the exocrine system, which secretes its chemicals using ducts. It derives from the Greek words "endo"...
and inflammation
Inflammation
Inflammation is part of the complex biological response of vascular tissues to harmful stimuli, such as pathogens, damaged cells, or irritants. Inflammation is a protective attempt by the organism to remove the injurious stimuli and to initiate the healing process...
. The synthesis
Chemical synthesis
In chemistry, chemical synthesis is purposeful execution of chemical reactions to get a product, or several products. This happens by physical and chemical manipulations usually involving one or more reactions...
of glycopeptides provides biological probes for researchers to elucidate glycan function in nature and products that have useful therapeutic and biotechnological applications.
N-Linked Glycans
N-linked glycans derive their name from the fact that the glycan is attached to an asparagineAsparagine
Asparagine is one of the 20 most common natural amino acids on Earth. It has carboxamide as the side-chain's functional group. It is not an essential amino acid...
(Asn, N) residue, and are amongst the most common linkages found in nature5. Although the majority of N-linked glycans take the form GlcNAc-β-Asn other less common structural linkages such as GlcNac-α-Asn and Glc-Asn have been observed.

O-Linked Glycans
O-Linked glycans are form by a linkage between an amino acid hydroxyl side chain (usually from serine or threonine) with the glycan. The majority of O-linked glycans take the form GlcNac-β-Ser/Thr or GalNac-α-Ser/Thr.
C-Linked Glycans
Of the three linkages the least common and least understood are C-linked glycans. The C-linkage refers to the covalent attachement of mannose to a tryptophan residue. An example of a C-linked glycan is α-mannosyl tryptophan.
Glycopeptide Synthesis
Several methods have been reported in the literature for the synthesis of glycopeptides. Of these methods the most common strategies are listed below.Solid Phase Peptide Synthesis (SPPS)
Within SPPS there exist two strategies for the synthesis of glycopeptides, linear and convergent assembly. Linear assembly relies on the synthesis of building blocks and then the use of SPPS to attach the building block together. An outline of this approach is illustrated below.

Native Chemical Ligation (NCL)
Native chemical ligationNative chemical ligation
Native chemical ligation or NCL is the most widely used form of chemical ligation, a technique for constructing a large polypeptide from two or more unprotected peptides. In native chemical ligation a peptide containing a C-terminal thioester reacts with another peptide containing an N-terminal...
, or NCL, is a convergent synthetic strategy based on the linear coupling of glycopeptide fragments. This technique makes use of the chemoselective reaction between a N-terminal cysteine residue on one peptide fragment with a thio-ester on the C-terminus of the other peptide fragment as illustrated below.

See also
- Glycopeptide antibiotics
- GlycosylationGlycosylationGlycosylation is the reaction in which a carbohydrate, i.e. a glycosyl donor, is attached to a hydroxyl or other functional group of another molecule . In biology glycosylation refers to the enzymatic process that attaches glycans to proteins, lipids, or other organic molecules...
- Peptide synthesisPeptide synthesisIn organic chemistry, peptide synthesis is the production of peptides, which are organic compounds in which multiple amino acids are linked via amide bonds which are also known as peptide bonds...
- Carbohydrate chemistryCarbohydrate chemistryCarbohydrate chemistry is a subdiscipline of chemistry primarily concerned with the synthesis, structure, and function of carbohydrate structures. Due to the general structure of carbohydrates, their synthesis is often preoccupied with the selective formation of glycosidic linkages and the...

