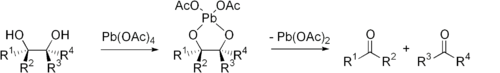Glycol cleavage
Encyclopedia
Glycol cleavage is a specific type of organic chemistry oxidation. The carbon–carbon bond in a (vicinal
diol
(glycol) is cleaved and replaced with two carbon–oxygen double bonds. Depending on the substitution pattern in the diol, either ketone
s or aldehyde
s may be formed.
Glycol cleavage is an important reaction in the laboratory because it is useful for determining the structures of sugars. After cleavage takes place the ketone and aldehyde fragments can be inspected and the location of the former hydroxyl groups ascertained.
(HIO4) and lead tetraacetate
(Pb(OAc)4) are the most common reagents used for glycol cleavage. These reactions involve cyclic intermediates
which then decompose to form ketones (if the R groups
are not H) or aldehydes (if one of the R groups is H).
Warm concentrated potassium permanganate
(KMnO4) will react with an alkene to form a glycol and will then immediately cleave the glycol to give stable ketones or oxidizable aldehydes. The aldehydes will react further to become carboxylic acid
s. Controlling the temperature and concentration of the reagent can keep the reaction from continuing past the formation of the glycol.
Glycol cleavage by periodic acid is called Malaprade periodic acid oxidation first reported by M. Malaprade in 1934 and also works with alpha aminoalcohols
Vicinal (chemistry)
In chemistry vicinal stands for any two functional groups bonded to two adjacent carbon atoms. For example the molecule 2,3-dibromobutane carries two vicinal bromine atoms and 1,3-dibromobutane does not....
diol
Diol
A diol or glycol is a chemical compound containing two hydroxyl groups A geminal diol has two hydroxyl groups bonded to the same atom...
(glycol) is cleaved and replaced with two carbon–oxygen double bonds. Depending on the substitution pattern in the diol, either ketone
Ketone
In organic chemistry, a ketone is an organic compound with the structure RCR', where R and R' can be a variety of atoms and groups of atoms. It features a carbonyl group bonded to two other carbon atoms. Many ketones are known and many are of great importance in industry and in biology...
s or aldehyde
Aldehyde
An aldehyde is an organic compound containing a formyl group. This functional group, with the structure R-CHO, consists of a carbonyl center bonded to hydrogen and an R group....
s may be formed.
Glycol cleavage is an important reaction in the laboratory because it is useful for determining the structures of sugars. After cleavage takes place the ketone and aldehyde fragments can be inspected and the location of the former hydroxyl groups ascertained.
Reagents
Periodic acidPeriodic acid
Periodic acid, or iodic acid is an oxoacid of iodine having chemical formula HIO4 or H5IO6.In dilute aqueous solution, periodic acid exists as discrete hydronium and metaperiodate ions. When more concentrated, orthoperiodic acid, H5IO6, is formed; this dissociates into hydronium and...
(HIO4) and lead tetraacetate
Lead(IV) acetate
Lead acetate or lead tetraacetate is a chemical compound with chemical formula Pb4 and is a lead salt of acetic acid. It is commercially available often stabilized with acetic acid....
(Pb(OAc)4) are the most common reagents used for glycol cleavage. These reactions involve cyclic intermediates
Reactive intermediate
In chemistry a reactive intermediate is a short-lived, high energy, highly reactive molecule. When generated in a chemical reaction it will quickly convert into a more stable molecule. Only in exceptional cases can these compounds be isolated and stored, e.g. low temperatures, matrix isolation...
which then decompose to form ketones (if the R groups
Side chain
In organic chemistry and biochemistry, a side chain is a chemical group that is attached to a core part of the molecule called "main chain" or backbone. The placeholder R is often used as a generic placeholder for alkyl group side chains in chemical structure diagrams. To indicate other non-carbon...
are not H) or aldehydes (if one of the R groups is H).
Warm concentrated potassium permanganate
Potassium permanganate
Potassium permanganate is an inorganic chemical compound with the formula KMnO4. It is a salt consisting of K+ and MnO4− ions. Formerly known as permanganate of potash or Condy's crystals, it is a strong oxidizing agent. It dissolves in water to give intensely purple solutions, the...
(KMnO4) will react with an alkene to form a glycol and will then immediately cleave the glycol to give stable ketones or oxidizable aldehydes. The aldehydes will react further to become carboxylic acid
Carboxylic acid
Carboxylic acids are organic acids characterized by the presence of at least one carboxyl group. The general formula of a carboxylic acid is R-COOH, where R is some monovalent functional group...
s. Controlling the temperature and concentration of the reagent can keep the reaction from continuing past the formation of the glycol.
Glycol cleavage by periodic acid is called Malaprade periodic acid oxidation first reported by M. Malaprade in 1934 and also works with alpha aminoalcohols