Glutamate carboxypeptidase II
Encyclopedia
Glutamate carboxypeptidase II (GCPII), also known as N-acetyl-L-aspartyl-L-glutamate peptidase I (NAALADase I), NAAG peptidase, or Prostate specific membrane antigen
(PSMA) is an enzyme
that in humans is encoded by the FOLH1 (folate hydrolase 1) gene
. Human GCPII contains 750 amino acids and weighs approximately 84 kD.
GCPII is a zinc metalloenzyme that resides in membranes. Most of the enzyme resides in the extracellular space. GCPII is a class II membrane glycoprotein
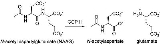
. It catalyzes the hydrolysis of N-acetylaspartylglutamate
(NAAG) to glutamate
and N-acetylaspartate (NAA) according to the reaction scheme to the right.
Neuroscientist primarily use the term NAALADase in their studies, while those studying folate metabolism use folate hydrolase, and those studying prostate cancer or oncology, PSMA. All of which refer to the same protein glutamate carboxypeptidase II.
Indeed the initial cloning of the cDNA encoding the gene expressing PSMA was accomplished with RNA from a prostate tumor cell line, LNCaP. PSMA shares homology with the transferrin receptor and undergoes endcytosis but the ligand for inducing internalization has not been identified. It was found that PSMA was the same as the membrane protein in the small intestine responsible for removal of gamma-linked glutamates from polygammaglutamate folate. This enables the freeing of folic acid, which then can be transported into the body for use as a vitamin. This resulted in the cloned genomic designation of PSMA as FOLH1 for folate hydrolase.
PSMA(FOLH1)+ folate polygammaglutamate(n 1-7)---> PSMA (FOLH1) + folate(poly)gammaglutamate(n-1) + glutamate continuing until releasing folate.
of the extracellular portion of GCPII –the protease, apical and C-terminal domains- collaborate in substrate recognition. The protease domain is a central seven-stranded mixed β-sheet. The β-sheet is flanked by 10 α-helices. The apical domain is located between the first and second strands of the central β-sheet of the protease domain. The apical domain creates a pocket that facilitates substrate binding. The C-terminal domain is an Up-Down-Up-Down four-helix bundle.
The central pocket is approximately 2 nanometres in depth and opens from the extracellular space to the active site. This active site contains two zinc ions. During inhibition, each acts as a ligand to an oxygen in 2-PMPA or phosphate.
There is also one calcium ion coordinated in GCPII, far from the active site. It has been proposed that calcium holds together the protease and apical domains.
In addition, ten glycosylation
sites have been identified in human GCPII. Glycosylation far from the catalytic domain still affects the ability of GCPII to hydrolyze NAAG.
In addition to the expression in the human prostate and prostate cancer, PSMA is also found to be highly expressed in tumor neovasculature but not normal vasculature of all types of solid tumors as kidney, breast, colon etc. In terms of imaging no non- tumor site such as normal kidney, small intestine, CNS has been imaged using second generation antibody imaging agents while sites even in bone are being detected with better sensitivity than with technetium scans and the tumors expressing PSMA in their neovasculature are also being imaged. Low molecular weight ligands exhibit different binding with imaging seen in the kidney of the mouse, however mouse has much higher levels of PSMA in kidney and brain than the human and in the mouse it was only the normal kidney and prostate tumors that were imaged and not other tissues, not even CNS suggesting the imprortance of the blood brain barrier. In kidney it is a subset of tubules that contain PSMA. Thus imaging studies will have to be performed in humans with the low molecular weight ligands to define their potential for imaging and targeting. Still, in terms of potential toxicities, knockout animals were normal on most tests, which reduces somewhat concerns about toxicity in targeting PSMA. In the CNS, PSMA is present only in a sub-set of glial cells, again suggesting that toxin trageting would likely have minimal toxicity to the host even if the blood brain barrier were not intact.
and glutamate. It does this through interaction with and activation of presynaptic group II mGluRs.ref name="Zhou_2005"/> Thus, in the presence of NAAG peptidase, the concentration of NAAG is kept in check, and glutamate and GABA, among other neurotransmitters, are not inhibited.
Researchers have been able to show that effective and selective GCPII inhibitors are able to decrease the brain's levels of glutamate and even provide protection from apoptosis or degradation of brain neurons in many animal models of stroke, amyotrophic lateral sclerosis, and neuropathic pain. This inhibition of these NAAG peptidases, sometimes referred to as NPs, are thought to provide this protection from apoptosis or degradation of brain neurons by elevating the concentrations of NAAG within the synapse of neurons. NAAG then reduces the release of glutamate while stimulating the release of some trophic factors from the glia cells in the central nervous system, resulting in the protection from apoptosis or degradation of brain neurons. It is important to note, however, that these NP inhibitors do not seem to have any effect on normal glutamate function. The NP inhibition is able to improve the naturally occurring regulation instead of activating or inhibiting receptors that would disrupt this process. Research has also shown that small-molecule-based NP inhibitors are beneficial in animal models that are relevant to neurodegenerative diseases. Some specific applications of this research include neuropathic and inflammatory pain, traumatic brain injury, ischemic stroke, schizophrenia, diabetic neuropathy, amyotrophic lateral sclerosis, as well as drug addiction. Previous research has found that drugs that are able to reduce glutamate transmission can relieve the neuropathic pain, although the resultant side-effects have limited a great deal of their clinical applications. Therefore, it appears that, since GCPII is exclusively recruited for the purpose of providing a glutamate source in hyperglutamatergic and excitotoxic conditions, this could be an alternative to avert these side-effects. More research findings have shown that the hydrolysis of NAAG is disrupted in schizophrenia, and they have shown that specific anatomical regions of the brain may even show discrete abnormalities in the GCP II synthesis, so NPs may also be therapeutic for patients suffering with schizophrenia. One major hurdle with using many of the potent GCPII inhibitors that have been prepared to date are typically highly polar compounds, which causes problems because they do not then penetrate the blood-brain barrier easily.
Due to the range of glutamate function and presence, it has been difficult to create glutamatergic drugs that do not negatively affect other necessary functions and cause unwanted side-effects. NAAG peptidase inhibition has offered the possibility for specific drug targeting.
is the third-leading cause of death and the leading cause of adult disability. It is thought that glutamate levels cause underlying ischemic damage during a stroke, and, thus, NAAG inhibition might be able to diminish this damage.
is a mental disorder that affects 1% of people throughout the world. It can be modeled by PCP in laboratory animals, and it has been shown that mGluR agonists have reduced the effects of the drug. NAAG is, such, an mGluR agonist. Thus, inhibition of the enzyme that reduces NAAG concentration, NAAG peptidase, could provide a practical treatment for reduction of schizophrenic symptoms.
Through the use of the NAAG peptidase inhibitor 2-PMPA, NAAG cleavage was inhibited and, with it, programmed DRG neuronal cell death in the presence of high glucose levels. The researchers have proposed that the cause of this is NAAG’s agonistic activity at mGluR3. In addition, NAAG also “prevented glucose-induced inhibition of neurite growth” (Berent- Spillson, et al. 2004). Overall, this makes GCPIII inhibition a clear model target for combating diabetic neuropathy.
In summary, the findings of multiple drug studies to conclude that:
Prostate specific membrane antigen
Prostate-specific membrane antigen is a type 2 integral membrane glycoproteinfound in prostate tissues and a few other tissues. It is a possible therapeutic target for prostate cancer. Although it is a marker for prostate cancer little is known about its function in the body...
(PSMA) is an enzyme
Enzyme
Enzymes are proteins that catalyze chemical reactions. In enzymatic reactions, the molecules at the beginning of the process, called substrates, are converted into different molecules, called products. Almost all chemical reactions in a biological cell need enzymes in order to occur at rates...
that in humans is encoded by the FOLH1 (folate hydrolase 1) gene
Gene
A gene is a molecular unit of heredity of a living organism. It is a name given to some stretches of DNA and RNA that code for a type of protein or for an RNA chain that has a function in the organism. Living beings depend on genes, as they specify all proteins and functional RNA chains...
. Human GCPII contains 750 amino acids and weighs approximately 84 kD.
GCPII is a zinc metalloenzyme that resides in membranes. Most of the enzyme resides in the extracellular space. GCPII is a class II membrane glycoprotein
Glycoprotein
Glycoproteins are proteins that contain oligosaccharide chains covalently attached to polypeptide side-chains. The carbohydrate is attached to the protein in a cotranslational or posttranslational modification. This process is known as glycosylation. In proteins that have segments extending...
. It catalyzes the hydrolysis of N-acetylaspartylglutamate
N-Acetylaspartylglutamate
N-Acetylaspartylglutamic acid is a neuropeptide that is the third-most-prevalent neurotransmitter in the mammalian nervous system. NAAG consists of N-acetylaspartic acid and glutamic acid coupled via a peptide bond...
(NAAG) to glutamate
Glutamic acid
Glutamic acid is one of the 20 proteinogenic amino acids, and its codons are GAA and GAG. It is a non-essential amino acid. The carboxylate anions and salts of glutamic acid are known as glutamates...
and N-acetylaspartate (NAA) according to the reaction scheme to the right.
Neuroscientist primarily use the term NAALADase in their studies, while those studying folate metabolism use folate hydrolase, and those studying prostate cancer or oncology, PSMA. All of which refer to the same protein glutamate carboxypeptidase II.
Discovery
GCPII is expressed in many tissues, including the prostate, kidney, the small intestine, and the central and peripheral nervous system.Indeed the initial cloning of the cDNA encoding the gene expressing PSMA was accomplished with RNA from a prostate tumor cell line, LNCaP. PSMA shares homology with the transferrin receptor and undergoes endcytosis but the ligand for inducing internalization has not been identified. It was found that PSMA was the same as the membrane protein in the small intestine responsible for removal of gamma-linked glutamates from polygammaglutamate folate. This enables the freeing of folic acid, which then can be transported into the body for use as a vitamin. This resulted in the cloned genomic designation of PSMA as FOLH1 for folate hydrolase.
PSMA(FOLH1)+ folate polygammaglutamate(n 1-7)---> PSMA (FOLH1) + folate(poly)gammaglutamate(n-1) + glutamate continuing until releasing folate.
Structure
The three domainsProtein domain
A protein domain is a part of protein sequence and structure that can evolve, function, and exist independently of the rest of the protein chain. Each domain forms a compact three-dimensional structure and often can be independently stable and folded. Many proteins consist of several structural...
of the extracellular portion of GCPII –the protease, apical and C-terminal domains- collaborate in substrate recognition. The protease domain is a central seven-stranded mixed β-sheet. The β-sheet is flanked by 10 α-helices. The apical domain is located between the first and second strands of the central β-sheet of the protease domain. The apical domain creates a pocket that facilitates substrate binding. The C-terminal domain is an Up-Down-Up-Down four-helix bundle.
The central pocket is approximately 2 nanometres in depth and opens from the extracellular space to the active site. This active site contains two zinc ions. During inhibition, each acts as a ligand to an oxygen in 2-PMPA or phosphate.
There is also one calcium ion coordinated in GCPII, far from the active site. It has been proposed that calcium holds together the protease and apical domains.
In addition, ten glycosylation
Glycosylation
Glycosylation is the reaction in which a carbohydrate, i.e. a glycosyl donor, is attached to a hydroxyl or other functional group of another molecule . In biology glycosylation refers to the enzymatic process that attaches glycans to proteins, lipids, or other organic molecules...
sites have been identified in human GCPII. Glycosylation far from the catalytic domain still affects the ability of GCPII to hydrolyze NAAG.
Enzyme kinetics
The hydrolysis of NAAG by GCPII obeys Michaelis-Menten kinetics calculated the binding constant (Km) for NAAG as approximately 130 nM and the turnover constant (kcat) as approxately 4 s-1. The apparent second-order rate constant is approximately 3 x 107 (M·s)-1.Role in prostate cancer
It was found that there were multiple potential start sites for PSMA as well as multiple alternative splice forms that vary in the type of membrane protein formed or having a cytosolic location and each form probably varies regarding caboxypeptidase activity given the restriction for enzymatic activity for PSMA. PSMA is strongly expressed in the human prostate being a hundredfold greater than the expression in most other tissues. In cancer it is upregulated in expression and has been called the second most up-regulated gene in prostate cancer being increased 8- 12 fold over the non cancerous prostate. Because of this high expression it is being developed as a target for therapy and imaging. In human prostate cancer the higher expressing tumors are associated with quicker time to progression and a greater percentage of patients suffering relapse. PSMA is the target of an approved imaging agent for prostate cancer, capromabpentide, PROSTASCINT. Second generation antibodies and low molecular weight ligands for imaging and therapy are being developed.In addition to the expression in the human prostate and prostate cancer, PSMA is also found to be highly expressed in tumor neovasculature but not normal vasculature of all types of solid tumors as kidney, breast, colon etc. In terms of imaging no non- tumor site such as normal kidney, small intestine, CNS has been imaged using second generation antibody imaging agents while sites even in bone are being detected with better sensitivity than with technetium scans and the tumors expressing PSMA in their neovasculature are also being imaged. Low molecular weight ligands exhibit different binding with imaging seen in the kidney of the mouse, however mouse has much higher levels of PSMA in kidney and brain than the human and in the mouse it was only the normal kidney and prostate tumors that were imaged and not other tissues, not even CNS suggesting the imprortance of the blood brain barrier. In kidney it is a subset of tubules that contain PSMA. Thus imaging studies will have to be performed in humans with the low molecular weight ligands to define their potential for imaging and targeting. Still, in terms of potential toxicities, knockout animals were normal on most tests, which reduces somewhat concerns about toxicity in targeting PSMA. In the CNS, PSMA is present only in a sub-set of glial cells, again suggesting that toxin trageting would likely have minimal toxicity to the host even if the blood brain barrier were not intact.
Neurotransmitter degradation
For those studying neural based diseases, NAAG is one of the three most prevalent neurotransmitters found in the central nervous system and when it catylizes the reaction to produce glutamate it is also producing another neurotransmitter. Glutamate is a common and abundant excitatory neurotransmitter in the central nervous system; although if there is too much glutamate transmission this can kill or at least damage neurons and has been implicated in many neurological diseases and disorders therefore the balance that NAAG peptidase contributes to is quite important.Function in the brain
GCPII has been shown to both indirectly and directly increase the concentration of glutamate in the extra cellular space. GCPII directly cleaves NAAG into NAA and glutamate. NAAG has been shown, in high concentration, to indirectly inhibit the release of neutrotransmitters, such as GABAGabâ
Gabâ or gabaa, for the people in many parts of the Philippines), is the concept of a non-human and non-divine, imminent retribution. A sort of negative karma, it is generally seen as an evil effect on a person because of their wrongdoings or transgressions...
and glutamate. It does this through interaction with and activation of presynaptic group II mGluRs.ref name="Zhou_2005"/> Thus, in the presence of NAAG peptidase, the concentration of NAAG is kept in check, and glutamate and GABA, among other neurotransmitters, are not inhibited.
Researchers have been able to show that effective and selective GCPII inhibitors are able to decrease the brain's levels of glutamate and even provide protection from apoptosis or degradation of brain neurons in many animal models of stroke, amyotrophic lateral sclerosis, and neuropathic pain. This inhibition of these NAAG peptidases, sometimes referred to as NPs, are thought to provide this protection from apoptosis or degradation of brain neurons by elevating the concentrations of NAAG within the synapse of neurons. NAAG then reduces the release of glutamate while stimulating the release of some trophic factors from the glia cells in the central nervous system, resulting in the protection from apoptosis or degradation of brain neurons. It is important to note, however, that these NP inhibitors do not seem to have any effect on normal glutamate function. The NP inhibition is able to improve the naturally occurring regulation instead of activating or inhibiting receptors that would disrupt this process. Research has also shown that small-molecule-based NP inhibitors are beneficial in animal models that are relevant to neurodegenerative diseases. Some specific applications of this research include neuropathic and inflammatory pain, traumatic brain injury, ischemic stroke, schizophrenia, diabetic neuropathy, amyotrophic lateral sclerosis, as well as drug addiction. Previous research has found that drugs that are able to reduce glutamate transmission can relieve the neuropathic pain, although the resultant side-effects have limited a great deal of their clinical applications. Therefore, it appears that, since GCPII is exclusively recruited for the purpose of providing a glutamate source in hyperglutamatergic and excitotoxic conditions, this could be an alternative to avert these side-effects. More research findings have shown that the hydrolysis of NAAG is disrupted in schizophrenia, and they have shown that specific anatomical regions of the brain may even show discrete abnormalities in the GCP II synthesis, so NPs may also be therapeutic for patients suffering with schizophrenia. One major hurdle with using many of the potent GCPII inhibitors that have been prepared to date are typically highly polar compounds, which causes problems because they do not then penetrate the blood-brain barrier easily.
Potential uses of NAAG peptidase inhibitors
Glutamate is the “primary excitatory neurotransmitter in the human nervous system”, participating in a multitude of brain functions. Over-stimulation and -activation of glutamate receptors as well as “disturbances in the cellular mechanisms that protect against the adverse consequences of physiological glutamate receptor activation” have been known to cause neuron damage and death, which have been associated with multiple neurological diseases.Due to the range of glutamate function and presence, it has been difficult to create glutamatergic drugs that do not negatively affect other necessary functions and cause unwanted side-effects. NAAG peptidase inhibition has offered the possibility for specific drug targeting.
Specific inhibitors
Since its promise for possible neurological disease therapy and specific drug targeting, NAAG peptidase inhibitors have been widely created and studied. A few small molecule examples are those that follow:- 2-PMPA and analogues
- Thiol and indole thiol derivatives
- Hydroxamate derivatives
- Conformationally constricted dipeptide mimetics
- PBDA- and urea-based inhibitors.

Neuropathic and inflammatory pain
Pain cause by injury to CNS or PNS has been associated with increase glutamate concentration. NAAG inhibition reduced glutamate presence and could, thus, diminish pain. (Neale JH et al., 2005). Nagel et al. used the inhibitor 2-PMPA to show the analgesic effect of NAAG peptidase inhibitions. This study followed one by Chen et al., which showed similar results.Head injury
Severe head injury (SHI) and traumatic brain injury (TBI) are widespread and have a tremendous impact. “They are the leading cause of death in children and young adults (<25 years) and account for a quarter of all deaths in the five to 15 years age group”. Following initial impact, glutamate levels rise and cause excitotoxic damage in a process that has been well characterized. With its ability to reduce glutamate levels, NAAG inhibition has shown promise in prevent neurological damage associated with SHI and TBI.Stroke
According to the National Stroke Association, strokeStroke
A stroke, previously known medically as a cerebrovascular accident , is the rapidly developing loss of brain function due to disturbance in the blood supply to the brain. This can be due to ischemia caused by blockage , or a hemorrhage...
is the third-leading cause of death and the leading cause of adult disability. It is thought that glutamate levels cause underlying ischemic damage during a stroke, and, thus, NAAG inhibition might be able to diminish this damage.
Schizophrenia
SchizophreniaSchizophrenia
Schizophrenia is a mental disorder characterized by a disintegration of thought processes and of emotional responsiveness. It most commonly manifests itself as auditory hallucinations, paranoid or bizarre delusions, or disorganized speech and thinking, and it is accompanied by significant social...
is a mental disorder that affects 1% of people throughout the world. It can be modeled by PCP in laboratory animals, and it has been shown that mGluR agonists have reduced the effects of the drug. NAAG is, such, an mGluR agonist. Thus, inhibition of the enzyme that reduces NAAG concentration, NAAG peptidase, could provide a practical treatment for reduction of schizophrenic symptoms.
Diabetic neuropathy
Diabetes can lead to damaged nerves, causing loss of sensation, pain, or, if autonomic nerves are associated, damage to the circulatory, reproductive, or digestive systems, among others. Over 60% of diabetic patients are said to have some form of neuropathy, however the severity ranges dramatically. Neuropathy not only directly causes harm and damage but also can indirectly lead to such problems as diabetic ulcerations, which in turn can lead to amputations. In fact, over half of all lower limb amputations in the United States are of patients with diabetes.Through the use of the NAAG peptidase inhibitor 2-PMPA, NAAG cleavage was inhibited and, with it, programmed DRG neuronal cell death in the presence of high glucose levels. The researchers have proposed that the cause of this is NAAG’s agonistic activity at mGluR3. In addition, NAAG also “prevented glucose-induced inhibition of neurite growth” (Berent- Spillson, et al. 2004). Overall, this makes GCPIII inhibition a clear model target for combating diabetic neuropathy.
Drug addiction
Schizophrenia, as previously described, is normally modeled in the laboratory through a PCP animal model. As GCPIII inhibition was shown to possibly limit schizophrenic behavior in this model, this suggests that GCPIII inhibition, thus, reduces the effect of PCP. In addition, the reward action of many drugs (cocaine, PCP, alcohol, nicotine, etc.) have been shown with increasing evidence to be related to glutamate levels, on which NAAG and GCPIII can have some regulatory effect.In summary, the findings of multiple drug studies to conclude that:
- NAAG/NP system might be involved in neuronal mechanisms regulating cue-induced cocaine craving, the development of cocaine seizure kindling, and management of opioid addiction and alcohol consumptive behaviour. Therefore, NP inhibitors could provide a novel therapy for such conditions.
Other diseases and disorders
NAAG inhibition has also been studied as a treatment against prostate cancer, ALS, and other neurodegenerative diseases such as Parkinson’s disease and Huntington’s disease.External links
- The MEROPSMeropsMerops may refer to:* Merops , a genus of bee-eaters.* MEROPS, an on-line database for peptidases.It may also refer to several figures from Greek mythology:* King of Ethiopia, husband of Clymene, who lay with Helios and bore Phaethon...
online database for peptidases and their inhibitors: M20.001 - Protein Data Bank: Protein Data Bank

