
FtsZ
Encyclopedia
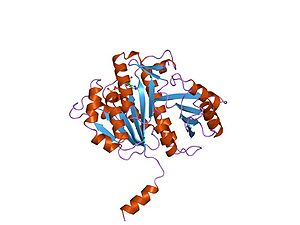
Protein
Proteins are biochemical compounds consisting of one or more polypeptides typically folded into a globular or fibrous form, facilitating a biological function. A polypeptide is a single linear polymer chain of amino acids bonded together by peptide bonds between the carboxyl and amino groups of...
encoded by the ftsZ gene
Gene
A gene is a molecular unit of heredity of a living organism. It is a name given to some stretches of DNA and RNA that code for a type of protein or for an RNA chain that has a function in the organism. Living beings depend on genes, as they specify all proteins and functional RNA chains...
that assembles into a ring at the future site of the septum
Septum
In anatomy, a septum is a wall, dividing a cavity or structure into smaller ones.-In human anatomy:...
of bacterial cell division
Cell division
Cell division is the process by which a parent cell divides into two or more daughter cells . Cell division is usually a small segment of a larger cell cycle. This type of cell division in eukaryotes is known as mitosis, and leaves the daughter cell capable of dividing again. The corresponding sort...
. This is a prokaryotic homologue
Homology (biology)
Homology forms the basis of organization for comparative biology. In 1843, Richard Owen defined homology as "the same organ in different animals under every variety of form and function". Organs as different as a bat's wing, a seal's flipper, a cat's paw and a human hand have a common underlying...
to the eukaryotic protein tubulin
Tubulin
Tubulin is one of several members of a small family of globular proteins. The most common members of the tubulin family are α-tubulin and β-tubulin, the proteins that make up microtubules. Each has a molecular weight of approximately 55 kiloDaltons. Microtubules are assembled from dimers of α- and...
. FtsZ has been named after "Filamenting temperature-sensitive mutant Z". The hypothesis was that cell division mutants of E. coli would grow as filaments due to the inability of the daughter cells to separate from one another.
History
Discovery of the bacterial cytoskeletonCytoskeleton
The cytoskeleton is a cellular "scaffolding" or "skeleton" contained within a cell's cytoplasm and is made out of protein. The cytoskeleton is present in all cells; it was once thought to be unique to eukaryotes, but recent research has identified the prokaryotic cytoskeleton...
is fairly recent. FtsZ was the first protein of the prokaryotic cytoskeleton
Prokaryotic cytoskeleton
The prokaryotic cytoskeleton is the collective name for all structural filaments in prokaryotes. It was once thought that prokaryotic cells did not possess cytoskeletons, but recent advances in visualization technology and structure determination have shown that filaments indeed exist in these cells...
to be identified.
The gene was discovered in the 1950s by Y. Hirota (:ja:廣田幸敬) and his colleagues in a screen for bacterial cell division mutants. In 1991 it was shown by Erfei Bi and Joseph Lutkenhaus that FtsZ assembled into the Z-ring.
Nuclear-encoded FtsZ in the moss Physcomitrella patens
Physcomitrella patens
Physcomitrella patens is a moss used as a model organism for studies on plant evolution, development and physiology.-Model organism:...
is required for chloroplast
Chloroplast
Chloroplasts are organelles found in plant cells and other eukaryotic organisms that conduct photosynthesis. Chloroplasts capture light energy to conserve free energy in the form of ATP and reduce NADP to NADPH through a complex set of processes called photosynthesis.Chloroplasts are green...
division and was the first identified protein essential for organelle
Organelle
In cell biology, an organelle is a specialized subunit within a cell that has a specific function, and is usually separately enclosed within its own lipid bilayer....
division in any eukaryote
Eukaryote
A eukaryote is an organism whose cells contain complex structures enclosed within membranes. Eukaryotes may more formally be referred to as the taxon Eukarya or Eukaryota. The defining membrane-bound structure that sets eukaryotic cells apart from prokaryotic cells is the nucleus, or nuclear...
.
Function
During cell divisionCell division
Cell division is the process by which a parent cell divides into two or more daughter cells . Cell division is usually a small segment of a larger cell cycle. This type of cell division in eukaryotes is known as mitosis, and leaves the daughter cell capable of dividing again. The corresponding sort...
, FtsZ is the first protein to move to the division site, and is essential for recruiting other proteins that produce a new cell wall
Cell wall
The cell wall is the tough, usually flexible but sometimes fairly rigid layer that surrounds some types of cells. It is located outside the cell membrane and provides these cells with structural support and protection, and also acts as a filtering mechanism. A major function of the cell wall is to...
between the dividing cells. FtsZ's role in cell division is analogous to that of actin
Actin
Actin is a globular, roughly 42-kDa moonlighting protein found in all eukaryotic cells where it may be present at concentrations of over 100 μM. It is also one of the most highly-conserved proteins, differing by no more than 20% in species as diverse as algae and humans...
in eukaryotic cell division, but, unlike the actin
Actin
Actin is a globular, roughly 42-kDa moonlighting protein found in all eukaryotic cells where it may be present at concentrations of over 100 μM. It is also one of the most highly-conserved proteins, differing by no more than 20% in species as diverse as algae and humans...
-myosin
Myosin
Myosins comprise a family of ATP-dependent motor proteins and are best known for their role in muscle contraction and their involvement in a wide range of other eukaryotic motility processes. They are responsible for actin-based motility. The term was originally used to describe a group of similar...
ring in eukaryotes, FtsZ has no known motor protein associated with it. The origin of the cytokinetic
Cytokinesis
Cytokinesis is the process in which the cytoplasm of a single eukaryotic cell is divided to form two daughter cells. It usually initiates during the late stages of mitosis, and sometimes meiosis, splitting a binucleate cell in two, to ensure that chromosome number is maintained from one generation...
force, thus, remains unclear, but it is believed that the localized synthesis of new cell wall produces at least part of this force. In liposomes Osawa (2009) showed FtsZ is capable of exerting a contractile force with no other proteins present.
Erickson (2009) proposed how the roles of tubulin-like proteins and actin-like proteins in cell division became reversed in an evolutionary mystery.
The use of the FtsZ ring in dividing chloroplast
Chloroplast
Chloroplasts are organelles found in plant cells and other eukaryotic organisms that conduct photosynthesis. Chloroplasts capture light energy to conserve free energy in the form of ATP and reduce NADP to NADPH through a complex set of processes called photosynthesis.Chloroplasts are green...
s and some mitochondria
Mitochondrion
In cell biology, a mitochondrion is a membrane-enclosed organelle found in most eukaryotic cells. These organelles range from 0.5 to 1.0 micrometers in diameter...
further establishes their prokaryotic ancestry. It is interesting to note that L-form bacteria
L-form bacteria
L-form bacteria, also known as L-phase bacteria, L-phase variants, and cell wall-deficient bacteria, are strains of bacteria that lack cell walls...
that lack a cell wall
Cell wall
The cell wall is the tough, usually flexible but sometimes fairly rigid layer that surrounds some types of cells. It is located outside the cell membrane and provides these cells with structural support and protection, and also acts as a filtering mechanism. A major function of the cell wall is to...
do not require FtsZ for division, which implies that bacteria may have retained components of an ancestral mode of cell division.
Much is known about the dynamic polymerization activities of tubulin
Tubulin
Tubulin is one of several members of a small family of globular proteins. The most common members of the tubulin family are α-tubulin and β-tubulin, the proteins that make up microtubules. Each has a molecular weight of approximately 55 kiloDaltons. Microtubules are assembled from dimers of α- and...
and microtubules, but little is known about these activities in FtsZ. While it is known that single-stranded tubulin
Tubulin
Tubulin is one of several members of a small family of globular proteins. The most common members of the tubulin family are α-tubulin and β-tubulin, the proteins that make up microtubules. Each has a molecular weight of approximately 55 kiloDaltons. Microtubules are assembled from dimers of α- and...
protofilaments form into 13 stranded microtubules, the multistranded structure of the FtsZ-containing Z-ring is not known. It is only speculated that the structure consists of overlapping protofilaments.
Recently, proteins similar to tubulin and FtsZ have been discovered in large plasmids found in Bacillus species. They are believed to function as components of segrosome
Segrosome
Segrosomes are protein complexes that ensure accurate segregation of plasmids or chromosomes during bacterial cell division.Just as higher forms of life have evolved a complex mitotic apparatus to partition duplicated DNA during cell division, bacteria require a specialized apparatus to partition...
s, which are multiprotein complexes that partition chromosomes/plasmid in bacteria. The plasmid homologs of tubulin/FtsZ seem to have conserved the ability to polymerize into filaments.
The Contractile Ring
FtsZ has the ability to bind to GTPGuanosine triphosphate
Guanosine-5'-triphosphate is a purine nucleoside triphosphate. It can act as a substrate for the synthesis of RNA during the transcription process...
and also exhibits a GTPase
GTPase
GTPases are a large family of hydrolase enzymes that can bind and hydrolyze guanosine triphosphate . The GTP binding and hydrolysis takes place in the highly conserved G domain common to all GTPases.-Functions:...
domain that allows it to hydrolyze GTP to GDP and a phosphate group. In vivo, FtsZ forms filaments with a repeating arrangement of subunits, all arranged head-to-tail. These filaments form a ring around the longitudinal midpoint, or septum, of the cell. This ring is called the Z-ring.
The GTP hydrolyzing activity of the protein is not essential to the formation of filaments or division. Mutants lacking the GTPase domain form twisted and disordered septa. These cells with irregular septa can still divide, although abnormally. It is unclear as to whether FtsZ actually provides the physical force that results in division or serves as a marker for other proteins to execute division.
In other models, FtsZ does not provide the contractile force but provides the cell a spatial scaffold for other proteins to execute the division of the cell. This is akin to the creating of a temporary structure by construction workers to access hard-to-reach places of a building. The temporary structure allows unfettered access and ensures that the workers can reach all places. If the temporary structure is not correctly built, the workers will not be able to reach certain places, and the building will be deficient.
The scaffold theory is supported by information that shows that the formation of the ring and localization to the membrane requires the concerted action of a number of accessory proteins. ZipA or the actin homologue FtsA permit initial FtsZ localization to the membrane. Following localization to the membrane, division proteins of the Fts family are recruited for ring assembly. Many of these proteins, such as FtsW, FtsK, and FtsQ are involved in stabilization of the Z ring and may also be active participants in the scission event.
Septal Localization and Intracellular Signaling
The formation of the Z-ring closely coincides with cellular processes associated with replication. Z-ring formation coincides with the termination of genome replication in E. coli and 70% of chromosomal replication in B. subtilis. The timing of Z-ring formation suggests the possibility of a spatial or temporal signal that permits the formation of FtsZ filaments. There currently exist several models and mechanisms that regulate Z-ring formation.The Min System
One model of Z-ring formation permits its formation only after a certain spatial signal that tells the cell that it is big enough to divide. FtsZ polymerization is closely linked to the Min family of proteins, which were all discovered as E. coli mutants that could not produce a properly localized septum. The Min proteins, which must prevent the FtsZ ring from being placed anywhere near the mid cell and nuclear material, are hypothesized to be involved in a spatial regulatory mechanism that links size increases prior to cell division to FtsZ polymerization in the middle of the cell.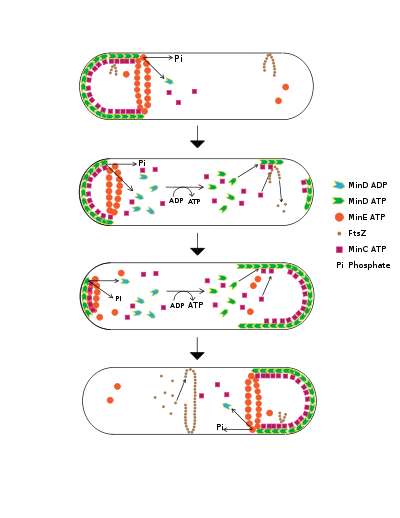
Mind
The concept of mind is understood in many different ways by many different traditions, ranging from panpsychism and animism to traditional and organized religious views, as well as secular and materialist philosophies. Most agree that minds are constituted by conscious experience and intelligent...
localizes to the membrane only at cell poles and contains an ATPase and an ATP-binding domain. The ATP-binding domain is important because MinD can bind to the membrane only if it is bound to ATP.
Once MinD is anchored in the membrane, it polymerizes, forming clusters of MinD. These clusters bind and then activate another protein called MinC
MINC
MINC is a data specification language written in the mid-1980s by a Princeton University graduate student named Lars Graf.It contains many of the syntactical capabilities of the C programming language, and can be used to implement simple procedural programs that can be executed by a runtime...
, which has activity only when bound by MinD. MinC serves as a FtsZ inhibitor that prevents FtsZ polymerization. The high concentration of a FtsZ polymerization inhibitor at the poles prevents FtsZ from initiating division at anywhere but the mid-cell.
MinE
Mine
Mine or mines can refer to:* Land mine, an anti-tank and anti-personnel weapon* Naval mine, an explosive device placed in water to destroy ships or submarines* Mining, extraction of mineral resources from the groundMine can also refer to:...
is involved in preventing the formation of MinCD complexes in the middle of the cell. MinE forms a ring near each cell pole. This ring is not like the Z-ring. Instead, it catalyzes the release of MinD from the membrane by activating MinD’s ATPase. This hydrolyzes the MinD’s bound ATP, preventing it form anchoring itself to the membrane.
MinE prevents the MinD/C complex from forming in the center but allows it to stay at the poles. Once the MinD/C complex is released, MinC becomes inactivated. This prevents MinC from deactivating FtsZ. As a consequence, this activity imparts regional specificity to Min localization. Thus, FtsZ can form only in the center, where there is no inhibitor. Mutations that prevent the formation of MinE rings result in MinCD polymers' extending well beyond the polar zones, preventing division from occurring.
MinD requires a nucleotide exchange step to re-bind to ATP so that it can reassociate with the membrane after MinE release. The time lapse results in a periodicity of Min association that may yield clues to a temporal signal linked to a spatial signal. In vivo observations show that the oscillation of Min proteins between cell poles occurs approximately every 50 seconds. Oscillation of Min proteins, however, is not necessary for all bacterial cell division systems. Bacillus subtilis has been shown to have static concentrations of MinC and MinD at the cell poles. This system still links cell size to the ability to form a septum via FtsZ and divide.
The dynamic behavior of the Min proteins has been reconstituted in vitro using an artificial lipid bilayer as mimic for the cell membrane. MinE and MinD self-organized into parallel and spiral protein waves by a reaction-diffusion-like mechanism.
.
Communicating Distress
FtsZ polymerization is also linked to stressors like DNA damage. DNA damage induces a variety of proteins to be manufactured, one of them called SulASula
-Places:Norway* Sula, Møre og Romsdal, an island and a municipality* Sula, Sogn og Fjordane, an island in Solund municipality* Sula, Sør-Trøndelag, an island group in Frøya municipality* Indre Sula and Ytre Sula, two mountains in Surnadal municipality...
. SulA prevents the polymerization and GTPase activity of FtsZ. SulA accomplishes this task by binding to self-recognizing FtsZ sites. By sequestering FtsZ, the cell can directly link DNA damage to inhibiting cell division.
Preventing DNA damage
Like SulA, there are other mechanisms that prevent cell division that would result in disrupted genetic information sent to daughter cells. So far, two proteins have been identified in E. coli and B. subtilis that prevent division over the nucleoid region: NocNoc
Noć is a village in the administrative district of Gmina Wierzbinek, within Konin County, Greater Poland Voivodeship, in west-central Poland. It lies approximately west of Wierzbinek, north of Konin, and east of the regional capital Poznań.The village has a population of 110.-References:...
and SlmA. Noc gene knockouts result in cells that divide without respect to the nucleoid
Nucleoid
The nucleoid is an irregularly-shaped region within the cell of a prokaryote that contains all or most of the genetic material. In contrast to the nucleus of a eukaryotic cell, it is not surrounded by a nuclear membrane. The genome of prokaryotic organisms generally is a circular, double-stranded...
region, resulting in its asymmetrical partitioning between the daughter cells. The mechanism is not well understood, but thought to involve sequestration of FtsZ, preventing polymerization
Polymerization
In polymer chemistry, polymerization is a process of reacting monomer molecules together in a chemical reaction to form three-dimensional networks or polymer chains...
over the nucleoid region. SlmA, like SulA, has been observed to sequester FtsZ, preventing the formation of the polymerized Z Ring over the nucleoid region.

