
Franz Hofmeister
Encyclopedia
Franz Hofmeister was an early protein scientist, and is famous for his studies of salts that influence the solubility and conformational stability of protein
s. Hofmeister was the first to propose that polypeptides were amino acid
s linked by peptide bond
s in 1902, although this model of protein primary structure
was independently and simultaneously conceived by Emil Fischer
.
, himself a student of Carl Lehmann. Hofmeister's Habilitationsschrift in 1879 concerned the peptic products of digestion.
Hofmeister became professor of Pharmacology in Prague in 1885, then moved to Strasbourg in 1896.
and tertiary structure
. Anions appear to have a larger effect than cations, and are usually ordered
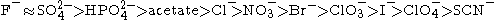
(This is a partial listing; many more salts have been studied.)
The order of cations is usually given as
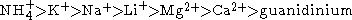
The mechanism of the Hofmeister series is not entirely clear, but seems to result mainly from effects on the solvent at higher salt concentrations (> 100 mM). Early members of the series increase solvent surface tension and decrease the solubility of nonpolar molecules (salt out); in effect, they strengthen the hydrophobic interaction. By contrast, later salts in the series increase the solubility of nonpolar molecules (salt in) and decrease the order in water; in effect, they weaken the hydrophobic effect
. However, these salts also interact directly with proteins (which are charged and have strong dipole moments) and may even bind specifically (e.g., phosphate and sulfate binding to ribonuclease A
). Ions that have a strong salting in
effect such as I− and SCN− are strong denaturants, because they salt in the peptide group, and thus interact much more strongly with the unfolded form of a protein than with its native form. Consequently, they pull the unfolding reaction. Moreover, they may have direct interactions with some standard hydrophobic molecules, e.g., benzene
.
. R=C-N-C=R bonds could be eliminated because it would imply a much larger number of carboxylate groups than is observed experimentally.
Hofmeister also argued for peptide bonds based on the biuret reaction observed with all proteins but never with free amino acids. Since biuret
has the formula NH2-CO-NH-CO-NH2, that suggested the presence of similar peptide bonds in proteins.
Protein
Proteins are biochemical compounds consisting of one or more polypeptides typically folded into a globular or fibrous form, facilitating a biological function. A polypeptide is a single linear polymer chain of amino acids bonded together by peptide bonds between the carboxyl and amino groups of...
s. Hofmeister was the first to propose that polypeptides were amino acid
Amino acid
Amino acids are molecules containing an amine group, a carboxylic acid group and a side-chain that varies between different amino acids. The key elements of an amino acid are carbon, hydrogen, oxygen, and nitrogen...
s linked by peptide bond
Peptide bond
This article is about the peptide link found within biological molecules, such as proteins. A similar article for synthetic molecules is being created...
s in 1902, although this model of protein primary structure
Primary structure
The primary structure of peptides and proteins refers to the linear sequence of its amino acid structural units. The term "primary structure" was first coined by Linderstrøm-Lang in 1951...
was independently and simultaneously conceived by Emil Fischer
Hermann Emil Fischer
Hermann Emil Fischer, Emil Fischer was a German chemist and 1902 recipient of the Nobel Prize in Chemistry. He discovered the Fischer esterification. He developed the Fischer projection, a symbolic way of drawing asymmetric carbon atoms.-Early years:Fischer was born in Euskirchen, near Cologne,...
.
Early life
Hofmeister's father was a doctor in Prague, where Hofmeister first began his studies, under the physiologist Karl Hugo HuppertKarl Hugo Huppert
Karl Hugo Huppert was a German chemist and physician .- Life and achievements :Karl Hugo Huppert, son of a wood turner and merchant, Christian Huppert, studied in Leipzig as a pupil of Karl Gotthelf Lehmann and in Jena. In 1960 he was appointed head of the chemical laboratory of the Jakob...
, himself a student of Carl Lehmann. Hofmeister's Habilitationsschrift in 1879 concerned the peptic products of digestion.
Hofmeister became professor of Pharmacology in Prague in 1885, then moved to Strasbourg in 1896.
The Hofmeister series
Hofmeister discovered a series of salts that have consistent effects on the solubility of proteins and (it was discovered later) on the stability of their secondarySecondary structure
In biochemistry and structural biology, secondary structure is the general three-dimensional form of local segments of biopolymers such as proteins and nucleic acids...
and tertiary structure
Tertiary structure
In biochemistry and molecular biology, the tertiary structure of a protein or any other macromolecule is its three-dimensional structure, as defined by the atomic coordinates.-Relationship to primary structure:...
. Anions appear to have a larger effect than cations, and are usually ordered
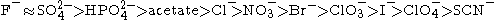
(This is a partial listing; many more salts have been studied.)
The order of cations is usually given as
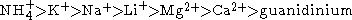
The mechanism of the Hofmeister series is not entirely clear, but seems to result mainly from effects on the solvent at higher salt concentrations (> 100 mM). Early members of the series increase solvent surface tension and decrease the solubility of nonpolar molecules (salt out); in effect, they strengthen the hydrophobic interaction. By contrast, later salts in the series increase the solubility of nonpolar molecules (salt in) and decrease the order in water; in effect, they weaken the hydrophobic effect
Hydrophobic effect
The hydrophobic effect is the observed tendency of nonpolar substances to aggregate in aqueous solution and exclude water molecules. The name, literally meaning "water-fearing," describes the segregation and apparent repulsion between water and nonpolar substances...
. However, these salts also interact directly with proteins (which are charged and have strong dipole moments) and may even bind specifically (e.g., phosphate and sulfate binding to ribonuclease A
Ribonuclease A
Ribonuclease A is a pancreatic ribonuclease that cleaves single-stranded RNA. Bovine pancreatic RNase A is one of the classic model systems of protein science.-History:...
). Ions that have a strong salting in
Salting in
Salting in refers to the effect where increasing the ionic strength of a solution increases the solubility of some solute . This effect tends to be observed at lower ionic strengths....
effect such as I− and SCN− are strong denaturants, because they salt in the peptide group, and thus interact much more strongly with the unfolded form of a protein than with its native form. Consequently, they pull the unfolding reaction. Moreover, they may have direct interactions with some standard hydrophobic molecules, e.g., benzene
Benzene
Benzene is an organic chemical compound. It is composed of 6 carbon atoms in a ring, with 1 hydrogen atom attached to each carbon atom, with the molecular formula C6H6....
.
Protein purification
The importance of the Hofmeister series to early protein work cannot be underestimated, since it provided the chief tool for purifying proteins (sulfate precipitation) over the next ~50 years, one that is still in use today. Hofmeister himself may have been the first to crystallize a protein, hen egg-white albumin. Repeated crystallization was a favorite purification technique in the early days of protein science, and was essential for its development.Proposal of protein primary structure
Hofmeister argued for peptide bonds by process of elimination. C-C, ether and ester bonds were unlikely considering the digestion by trypsinTrypsin
Trypsin is a serine protease found in the digestive system of many vertebrates, where it hydrolyses proteins. Trypsin is produced in the pancreas as the inactive proenzyme trypsinogen. Trypsin cleaves peptide chains mainly at the carboxyl side of the amino acids lysine or arginine, except when...
. R=C-N-C=R bonds could be eliminated because it would imply a much larger number of carboxylate groups than is observed experimentally.
Hofmeister also argued for peptide bonds based on the biuret reaction observed with all proteins but never with free amino acids. Since biuret
Biuret
Biuret is a chemical compound with the chemical formula H2NCNHCNH2. It is the result of condensation of two molecules of urea and is a problematic impurity in urea-based fertilizers. This white solid is soluble in hot water....
has the formula NH2-CO-NH-CO-NH2, that suggested the presence of similar peptide bonds in proteins.
See also
- primary structurePrimary structureThe primary structure of peptides and proteins refers to the linear sequence of its amino acid structural units. The term "primary structure" was first coined by Linderstrøm-Lang in 1951...
- peptide bondPeptide bondThis article is about the peptide link found within biological molecules, such as proteins. A similar article for synthetic molecules is being created...
- Picture of Hofmeister at Science and Society site, UK

