
Flavanone 3-dioxygenase
Encyclopedia
In enzymology, a flavanone 3-dioxygenase is an enzyme
that catalyzes
the chemical reaction
The 3 substrates
of this enzyme are flavanone
, 2-oxoglutarate, and O2
, whereas its 3 products
are dihydroflavonol, succinate, and CO2
.
This enzyme belongs to the family of oxidoreductase
s, specifically those acting on paired donors, with O2 as oxidant and incorporation or reduction of oxygen. The oxygen incorporated need not be derived from O2 with 2-oxoglutarate as one donor, and incorporation of one atom o oxygen into each donor. The systematic name of this enzyme class is flavanone,2-oxoglutarate:oxygen oxidoreductase (3-hydroxylating). Other names in common use include naringenin 3-hydroxylase, flavanone 3-hydroxylase, flavanone 3beta-hydroxylase, flavanone synthase I, (2S)-flavanone 3-hydroxylase, and naringenin,2-oxoglutarate:oxygen oxidoreductase (3-hydroxylating). This enzyme participates in flavonoid biosynthesis
. It has 2 cofactors
: iron
, and Ascorbate.
Enzyme
Enzymes are proteins that catalyze chemical reactions. In enzymatic reactions, the molecules at the beginning of the process, called substrates, are converted into different molecules, called products. Almost all chemical reactions in a biological cell need enzymes in order to occur at rates...
that catalyzes
Catalysis
Catalysis is the change in rate of a chemical reaction due to the participation of a substance called a catalyst. Unlike other reagents that participate in the chemical reaction, a catalyst is not consumed by the reaction itself. A catalyst may participate in multiple chemical transformations....
the chemical reaction
Chemical reaction
A chemical reaction is a process that leads to the transformation of one set of chemical substances to another. Chemical reactions can be either spontaneous, requiring no input of energy, or non-spontaneous, typically following the input of some type of energy, such as heat, light or electricity...
- a flavanone + 2-oxoglutarate + O2
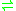 a dihydroflavonol + succinate + CO2
a dihydroflavonol + succinate + CO2
The 3 substrates
Substrate (biochemistry)
In biochemistry, a substrate is a molecule upon which an enzyme acts. Enzymes catalyze chemical reactions involving the substrate. In the case of a single substrate, the substrate binds with the enzyme active site, and an enzyme-substrate complex is formed. The substrate is transformed into one or...
of this enzyme are flavanone
Flavanone
The flavanones are a type of flavonoids. They are generally glycosylated by a disaccharide at position seven to give flavanone glycosides.-List of flavanones:* Butin* Eriodictyol* Hesperetin* Hesperidin* Homoeriodictyol* Isosakuranetin* Naringenin...
, 2-oxoglutarate, and O2
Oxygen
Oxygen is the element with atomic number 8 and represented by the symbol O. Its name derives from the Greek roots ὀξύς and -γενής , because at the time of naming, it was mistakenly thought that all acids required oxygen in their composition...
, whereas its 3 products
Product (chemistry)
Product are formed during chemical reactions as reagents are consumed. Products have lower energy than the reagents and are produced during the reaction according to the second law of thermodynamics. The released energy comes from changes in chemical bonds between atoms in reagent molecules and...
are dihydroflavonol, succinate, and CO2
Carbon dioxide
Carbon dioxide is a naturally occurring chemical compound composed of two oxygen atoms covalently bonded to a single carbon atom...
.
This enzyme belongs to the family of oxidoreductase
Oxidoreductase
In biochemistry, an oxidoreductase is an enzyme that catalyzes the transfer of electrons from one molecule to another...
s, specifically those acting on paired donors, with O2 as oxidant and incorporation or reduction of oxygen. The oxygen incorporated need not be derived from O2 with 2-oxoglutarate as one donor, and incorporation of one atom o oxygen into each donor. The systematic name of this enzyme class is flavanone,2-oxoglutarate:oxygen oxidoreductase (3-hydroxylating). Other names in common use include naringenin 3-hydroxylase, flavanone 3-hydroxylase, flavanone 3beta-hydroxylase, flavanone synthase I, (2S)-flavanone 3-hydroxylase, and naringenin,2-oxoglutarate:oxygen oxidoreductase (3-hydroxylating). This enzyme participates in flavonoid biosynthesis
Flavonoid biosynthesis
Flavonoids are synthesized by the phenylpropanoid metabolic pathway in which the amino acid phenylalanine is used to produce 4-coumaroyl-CoA. This can be combined with malonyl-CoA to yield the true backbone of flavonoids, a group of compounds called chalcones, which contain two phenyl rings...
. It has 2 cofactors
Cofactor (biochemistry)
A cofactor is a non-protein chemical compound that is bound to a protein and is required for the protein's biological activity. These proteins are commonly enzymes, and cofactors can be considered "helper molecules" that assist in biochemical transformations....
: iron
Iron
Iron is a chemical element with the symbol Fe and atomic number 26. It is a metal in the first transition series. It is the most common element forming the planet Earth as a whole, forming much of Earth's outer and inner core. It is the fourth most common element in the Earth's crust...
, and Ascorbate.

