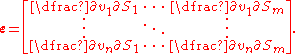Elasticity Coefficient
Encyclopedia
Elasticity Coefficients are used in Physics, Economics, Chemistry, or more generally in mathematics as a definition of point elasticity: the article below applies to Chemical/Biochemical Elasticity Coefficients.
The rate of a chemical reaction is influenced by many different factors, such as temperature, pH
, reactant and product
concentrations and other effectors. The degree to which these factors change the reaction rate is described by the elasticity coefficient. This coefficient is defined as follows:
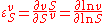
where denotes the reaction rate and
denotes the reaction rate and 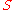 denotes the substrate
denotes the substrate
concentration. The partial derivative
in the definition indicates that the elasticity is measured with respect to changes in a factor S while keeping all other factors constant. The most common factors include substrates, products and effectors. The scaling of the coefficient ensures that it is dimensionless and independent of the units used to measure the reaction rate and magnitude of the factor. The elasticity coefficient is an integral part of Metabolic control analysis
and was introduced in the early 1970s and possibly earlier by Henrik Kacser and Burns6 in Edinburgh and Heinrich and Rapoport8 in Berlin.
The elasticity concept has also been described by other authors, most notably Savageau8 in Michigan and Clarke9 at Edmonton. In the late 1960s Michael Savageau8 developed an innovative approach called Biochemical systems theory
that uses power-law expansions to approximate the nonlinearities in biochemical kinetics. The theory is very similar to Metabolic control analysis
and has been very successfully and extensively used to study the properties of different feedback and other regulatory structures in cellular networks. The power-law expansions used in the analysis invoke coefficients called kinetic orders which are equivalent to the elasticity coefficients.
Bruce Clarke9 in the early 1970s developed a sophisticated theory on analyzing the dynamic stability in chemical networks. As part of his analysis Clarke also introduced the notion of kinetic orders and a power-law approximation that was somewhat similar to Savageau's power-law expansions. Clarke's approach relied heavily on certain structural characteristics of networks, called extreme currents (also called elementary modes in biochemical systems). Clarke's kinetic orders are also equivalent to elasticities.
The fact that different groups independently introduced the same concept implies that elasticities, or their equivalent, kinetic orders, are most likely a fundamental concept in the analysis of complex biochemical or chemical systems.
it is possible for example to determine the elasticity of an arbitrary rate law by differentiating the rate law by the independent variable and scaling. For example the elasticity coefficient for a mass-action rate law such as:
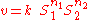
where is the Reaction rate
is the Reaction rate
,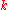 the Reaction rate constant,
the Reaction rate constant, 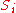 is the ith chemical species involved in the reaction and
is the ith chemical species involved in the reaction and 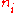 the ith reaction order, then the elasticity,
the ith reaction order, then the elasticity, 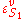 can be obtained by differentiating the rate law with respect to
can be obtained by differentiating the rate law with respect to 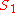 and scaling:
and scaling:

That is the elasticity for a mass-action rate law is equal to the Order of reaction of the species.
Elasticities can also be derived for more complex rate laws such as the Michaelis-Menten rate law. If
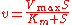
then it can be easily shown than
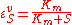
This equation illustrates the idea that elasticities need not be constants (as with mass-action laws) but can be a function
of the reactant concentration. In this case the elasticity approaches unity at low reactant concentration (S) and zero at high reactant concentration.
For the reversible Michaelis-Menten rate law:
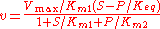
where is the forward
is the forward  ,
,  the forward
the forward 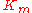 ,
, 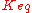 the equilibrium constant and
the equilibrium constant and  the reverse
the reverse 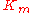 , two elasticity coefficients can be calculated, one with respect to S and another with respect to P. Thus:
, two elasticity coefficients can be calculated, one with respect to S and another with respect to P. Thus:
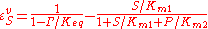
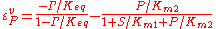
where is the mass-action ratio, that is
is the mass-action ratio, that is 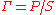 . Note that when P = 0, the equations reduce to the case for the irreversible Michaelis-Menten law.
. Note that when P = 0, the equations reduce to the case for the irreversible Michaelis-Menten law.
As a final example, consider the Hill equation:
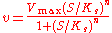
where n is the Hill coefficient and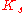 is the half-saturation coefficient (cf. Michaelis-Menten rate law), then the elasticity coefficient is given by:
is the half-saturation coefficient (cf. Michaelis-Menten rate law), then the elasticity coefficient is given by:
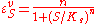
Note that at low S the elasticity approaches n. At high S the elasticity approaches zero. This means the elasticity is bounded between zero and the Hill coefficient.
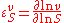
differentiating in log space is an obvious approach. Logarithmic differentiation is particularly convenient in algebra software such as Mathematica or Maple, where logarithmic differentiation rules can be defined.
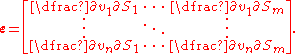
The rate of a chemical reaction is influenced by many different factors, such as temperature, pH
PH
In chemistry, pH is a measure of the acidity or basicity of an aqueous solution. Pure water is said to be neutral, with a pH close to 7.0 at . Solutions with a pH less than 7 are said to be acidic and solutions with a pH greater than 7 are basic or alkaline...
, reactant and product
Product (chemistry)
Product are formed during chemical reactions as reagents are consumed. Products have lower energy than the reagents and are produced during the reaction according to the second law of thermodynamics. The released energy comes from changes in chemical bonds between atoms in reagent molecules and...
concentrations and other effectors. The degree to which these factors change the reaction rate is described by the elasticity coefficient. This coefficient is defined as follows:
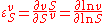
where
 denotes the reaction rate and
denotes the reaction rate and 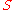 denotes the substrate
denotes the substrateSubstrate (chemistry)
In chemistry, a substrate is the chemical species being observed, which reacts with a reagent. This term is highly context-dependent. In particular, in biochemistry, an enzyme substrate is the material upon which an enzyme acts....
concentration. The partial derivative
Partial derivative
In mathematics, a partial derivative of a function of several variables is its derivative with respect to one of those variables, with the others held constant...
in the definition indicates that the elasticity is measured with respect to changes in a factor S while keeping all other factors constant. The most common factors include substrates, products and effectors. The scaling of the coefficient ensures that it is dimensionless and independent of the units used to measure the reaction rate and magnitude of the factor. The elasticity coefficient is an integral part of Metabolic control analysis
Metabolic control analysis
Metabolic control analysis is a mathematical framework for describingmetabolic, signaling and genetic pathways. MCA quantifies how variables,such as fluxes and species concentrations, depend on network parameters....
and was introduced in the early 1970s and possibly earlier by Henrik Kacser and Burns6 in Edinburgh and Heinrich and Rapoport8 in Berlin.
The elasticity concept has also been described by other authors, most notably Savageau8 in Michigan and Clarke9 at Edmonton. In the late 1960s Michael Savageau8 developed an innovative approach called Biochemical systems theory
Biochemical systems theory
Biochemical systems theory is a mathematical modelling framework for biochemical systems, based on ordinary differential equations , in which biochemical processes are represented using power-law expansions in the variables of the system....
that uses power-law expansions to approximate the nonlinearities in biochemical kinetics. The theory is very similar to Metabolic control analysis
Metabolic control analysis
Metabolic control analysis is a mathematical framework for describingmetabolic, signaling and genetic pathways. MCA quantifies how variables,such as fluxes and species concentrations, depend on network parameters....
and has been very successfully and extensively used to study the properties of different feedback and other regulatory structures in cellular networks. The power-law expansions used in the analysis invoke coefficients called kinetic orders which are equivalent to the elasticity coefficients.
Bruce Clarke9 in the early 1970s developed a sophisticated theory on analyzing the dynamic stability in chemical networks. As part of his analysis Clarke also introduced the notion of kinetic orders and a power-law approximation that was somewhat similar to Savageau's power-law expansions. Clarke's approach relied heavily on certain structural characteristics of networks, called extreme currents (also called elementary modes in biochemical systems). Clarke's kinetic orders are also equivalent to elasticities.
The fact that different groups independently introduced the same concept implies that elasticities, or their equivalent, kinetic orders, are most likely a fundamental concept in the analysis of complex biochemical or chemical systems.
Calculating Elasticity Coefficients
Elasticity coefficients can be calculated in various ways, either numerically or algebraically.Algebraic Calculation of Elasticity Coefficients
Given the definition of the elasticity in terms of a Partial derivativePartial derivative
In mathematics, a partial derivative of a function of several variables is its derivative with respect to one of those variables, with the others held constant...
it is possible for example to determine the elasticity of an arbitrary rate law by differentiating the rate law by the independent variable and scaling. For example the elasticity coefficient for a mass-action rate law such as:
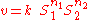
where
 is the Reaction rate
is the Reaction rateReaction rate
The reaction rate or speed of reaction for a reactant or product in a particular reaction is intuitively defined as how fast or slow a reaction takes place...
,
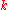 the Reaction rate constant,
the Reaction rate constant, 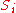 is the ith chemical species involved in the reaction and
is the ith chemical species involved in the reaction and 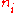 the ith reaction order, then the elasticity,
the ith reaction order, then the elasticity, 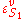 can be obtained by differentiating the rate law with respect to
can be obtained by differentiating the rate law with respect to 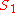 and scaling:
and scaling:
That is the elasticity for a mass-action rate law is equal to the Order of reaction of the species.
Elasticities can also be derived for more complex rate laws such as the Michaelis-Menten rate law. If
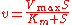
then it can be easily shown than
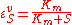
This equation illustrates the idea that elasticities need not be constants (as with mass-action laws) but can be a function
of the reactant concentration. In this case the elasticity approaches unity at low reactant concentration (S) and zero at high reactant concentration.
For the reversible Michaelis-Menten rate law:
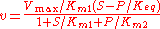
where
 is the forward
is the forward  ,
,  the forward
the forward 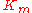 ,
, 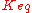 the equilibrium constant and
the equilibrium constant and  the reverse
the reverse 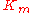 , two elasticity coefficients can be calculated, one with respect to S and another with respect to P. Thus:
, two elasticity coefficients can be calculated, one with respect to S and another with respect to P. Thus: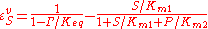
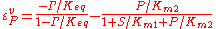
where
 is the mass-action ratio, that is
is the mass-action ratio, that is 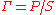 . Note that when P = 0, the equations reduce to the case for the irreversible Michaelis-Menten law.
. Note that when P = 0, the equations reduce to the case for the irreversible Michaelis-Menten law.As a final example, consider the Hill equation:
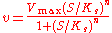
where n is the Hill coefficient and
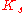 is the half-saturation coefficient (cf. Michaelis-Menten rate law), then the elasticity coefficient is given by:
is the half-saturation coefficient (cf. Michaelis-Menten rate law), then the elasticity coefficient is given by: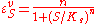
Note that at low S the elasticity approaches n. At high S the elasticity approaches zero. This means the elasticity is bounded between zero and the Hill coefficient.
Differentiating in Log Space5
An approach that is amenable to algebraic calculation by computer algebra methods is to differentiate in log space. Since the elasticity can be defined logarithmically, that is: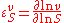
differentiating in log space is an obvious approach. Logarithmic differentiation is particularly convenient in algebra software such as Mathematica or Maple, where logarithmic differentiation rules can be defined.
Numerical Calculation of Elasticity Coefficients
Elasticities coefficient can also be computed numerically, something that is often done in simulation software.Elasticity Matrix
The unscaled elasticities are often depicted in matrix form, called the elasticity matrix. Given a network with m molecular species and n reactions, then the elasticity matrix is defined as: