
Edman degradation
Encyclopedia
Edman degradation, developed by Pehr Edman
, is a method of sequencing
amino acids in a peptide
. In this method, the amino-terminal residue
is labeled and cleaved from the peptide without disrupting the peptide bonds between other amino acid residues.
 Phenylisothiocyanate
Phenylisothiocyanate
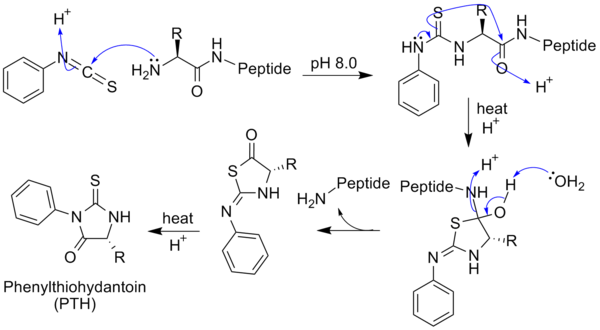
is reacted with an uncharged terminal amino group, under mildly alkaline conditions, to form a cyclical phenylthiocarbamoyl derivative. Then, under acidic conditions, this derivative of the terminal amino acid is cleaved as a thiazolinone derivative. The thiazolinone amino acid is then selectively extracted into an organic solvent and treated with acid to form the more stable phenylthiohydantoin (PTH)- amino acid derivative that can be identified by using chromatography
or electrophoresis
. This procedure can then be repeated again to identify the next amino acid. A major drawback to this technique is that the peptides being sequenced in this manner cannot have more than 50 to 60 residues (and in practice, under 30). The peptide length is limited due to the cyclical derivatization not always going to completion. The derivatization problem can be resolved by cleaving large peptides into smaller peptides before proceeding with the reaction. It is able to accurately sequence
up to 30 amino acids with modern machines capable of over 99% efficiency per amino acid
. An advantage of the Edman degradation is that it only uses 10 - 100 pico-moles of peptide
for the sequencing process. Edman degradation reaction is automated to speed up the process.
Pehr Edman
Pehr Victor Edman was a Swedish biochemist. He developed a method for sequencing proteins, the Edman degradation.- Early life :...
, is a method of sequencing
Protein sequencing
Protein sequencing is a technique to determine the amino acid sequence of a protein, as well as which conformation the protein adopts and the extent to which it is complexed with any non-peptide molecules...
amino acids in a peptide
Peptide
Peptides are short polymers of amino acid monomers linked by peptide bonds. They are distinguished from proteins on the basis of size, typically containing less than 50 monomer units. The shortest peptides are dipeptides, consisting of two amino acids joined by a single peptide bond...
. In this method, the amino-terminal residue
Residue (chemistry)
In chemistry, residue is the material remaining after a distillation or an evaporation, or to a portion of a larger molecule, such as a methyl group. It may also refer to the undesired byproducts of a reaction....
is labeled and cleaved from the peptide without disrupting the peptide bonds between other amino acid residues.
Mechanism

Isothiocyanate
Isothiocyanate is the chemical group –N=C=S, formed by substituting sulfur for oxygen in the isocyanate group. Many natural isothiocyanates from plants are produced by enzymatic conversion of metabolites called glucosinolates. These natural isothiocyanates, such as allyl isothiocyanate, are also...
is reacted with an uncharged terminal amino group, under mildly alkaline conditions, to form a cyclical phenylthiocarbamoyl derivative. Then, under acidic conditions, this derivative of the terminal amino acid is cleaved as a thiazolinone derivative. The thiazolinone amino acid is then selectively extracted into an organic solvent and treated with acid to form the more stable phenylthiohydantoin (PTH)- amino acid derivative that can be identified by using chromatography
Chromatography
Chromatography is the collective term for a set of laboratory techniques for the separation of mixtures....
or electrophoresis
Electrophoresis
Electrophoresis, also called cataphoresis, is the motion of dispersed particles relative to a fluid under the influence of a spatially uniform electric field. This electrokinetic phenomenon was observed for the first time in 1807 by Reuss , who noticed that the application of a constant electric...
. This procedure can then be repeated again to identify the next amino acid. A major drawback to this technique is that the peptides being sequenced in this manner cannot have more than 50 to 60 residues (and in practice, under 30). The peptide length is limited due to the cyclical derivatization not always going to completion. The derivatization problem can be resolved by cleaving large peptides into smaller peptides before proceeding with the reaction. It is able to accurately sequence
Sequence
In mathematics, a sequence is an ordered list of objects . Like a set, it contains members , and the number of terms is called the length of the sequence. Unlike a set, order matters, and exactly the same elements can appear multiple times at different positions in the sequence...
up to 30 amino acids with modern machines capable of over 99% efficiency per amino acid
Amino acid
Amino acids are molecules containing an amine group, a carboxylic acid group and a side-chain that varies between different amino acids. The key elements of an amino acid are carbon, hydrogen, oxygen, and nitrogen...
. An advantage of the Edman degradation is that it only uses 10 - 100 pico-moles of peptide
Peptide
Peptides are short polymers of amino acid monomers linked by peptide bonds. They are distinguished from proteins on the basis of size, typically containing less than 50 monomer units. The shortest peptides are dipeptides, consisting of two amino acids joined by a single peptide bond...
for the sequencing process. Edman degradation reaction is automated to speed up the process.

