Dubnium
Encyclopedia
The Soviet team proposed the name nielsbohrium (Ns) in honor of the Danish nuclear physicist Niels Bohr
. The American team proposed that the new element should be named hahnium (Ha), in honor of the late German
chemist Otto Hahn
. Consequently hahnium was the name that most American and Western European scientists used and appears in many papers published at the time, and nielsbohrium was used in the Soviet Union and Eastern Bloc
countries.
An element naming controversy
erupted between the two groups. The International Union of Pure and Applied Chemistry
(IUPAC) thus adopted unnilpentium (Unp) as a temporary, systematic element name
. Attempting to resolve the issue, in 1994, the IUPAC proposed the name joliotium (Jl), after the French physicist Frédéric Joliot-Curie
, which was originally proposed by Soviet team for element 102, later named nobelium
. The two principal claimants still disagreed about the names of elements 104-106. However, in 1997 they resolved the dispute and adopted the current name, dubnium (Db), after the Russia
n town of Dubna
, the location of the Joint Institute for Nuclear Research
. It was argued by IUPAC that the Berkeley laboratory had already been recognized several times in the naming of elements (i.e., berkelium
, californium
, americium
) and that the acceptance of the names rutherfordium
and seaborgium
for elements 104 and 106 should be offset by recognizing the Russian team's contributions to the discovery of elements 104, 105 and 106.
, niobium
and tantalum
. Because it is positioned right below tantalum, it may also be called eka-tantalum. All the members of the group readily portray their oxidation state of +5 and the state becomes more stable as the group is descended. Thus dubnium is expected to form a stable +5 state. For this group, +4 and +3 states are also known for the heavier members and dubnium may also form these reducing oxidation states.
In an extrapolation of the chemistries from niobium
and tantalum
, dubnium should react with oxygen to form an inert pentoxide, Db2O5. In alkali, the formation of an orthodubnate complex, DbO43−, is expected.
Reaction with the halogens should readily form the pentahalides, DbX5. The pentachlorides of niobium and tantalum exist as volatile solids or monomeric trigonal bipyramidal molecules in the vapour phase. Thus, DbCl5 is expected to be a volatile solid. Similarly, the pentafluoride, DbF5, should be even more volatile.
Hydrolysis of the halides is known to readily form the oxyhalides, MOX3. Thus the halides DbX5 should react with water to form DbOX3.
The reaction with fluoride ion is also well known for the lighter homologues and dubnium is expected to form a range of fluoro-complexes. In particular, reaction of the pentafluoride with HF should form a hexafluorodubnate ion, DbF6–. Excess fluoride should lead to DbF72– and DbOF52–. If eka-tantalum properties are portrayed, higher concentrations of fluoride should ultimately form DbF83– since NbF83– is not known.
, tantalum
and dubnium radioisotopes. The results have indicated the formation of typical group 5 halides and oxyhalides, namely DbCl5, DbBr5, DbOCl3 and DbOBr3. Reports on these early experiments usually refer to dubnium as hahnium.
209Bi(50Ti,xn)259-xDb (x=1,2,3)
The first attempts to synthesise dubnium using cold fusion reactions were performed in 1976 by the team at FLNR, Dubna using the above reaction. They were able to detect a 5 s spontaneous fission
(SF) activity which they assigned to 257Db. This assignment was later corrected to 258Db.
In 1981, the team at GSI studied this reaction using the improved technique of correlation of genetic parent-daughter decays. They were able to positively identify 258Db, the product from the 1n neutron evaporation channel.
In 1983, the team at Dubna revisited the reaction using the method of identification of a descendant using chemical separation. They succeeded in measuring alpha decays from known descendants of the decay chain beginning with 258Db. This was taken as providing some evidence for the formation of dubnium nuclei.
The team at GSI revisited the reaction in 1985 and were able to detect 10 atoms of 257Db.
After a significant upgrade of their facilities in 1993, in 2000 the team measured 120 decays of 257Db, 16 decays of 256Db and decay of 258Db in the measurement of the 1n, 2n and 3n excitation functions. The data gathered for 257Db allowed a first spectroscopic study of this isotope and identified an isomer, 257mDb, and a first determination of a decay level structure for 257Db.
The reaction was used in spectroscopic studies of isotopes of mendelevium
and einsteinium
in 2003-2004.
209Bi(49Ti,xn)258-xDb (x=2?)
This reaction was studied by Yuri Oganessian and the team at Dubna in 1983. They observed a 2.6 s SF activity tentatively assigned to 256Db. Later results suggest a possible reassignment to 256Rf, resulting from the ~30% EC branch in 256Db.
209Bi(48Ti,xn)257-xDb (x=1?)
This reaction was studied by Yuri Oganessian and the team at Dubna in 1983. They observed a 1.6 s activity with a ~80% alpha branch with a ~20% SF branch. The activity was tentatively assigned to 255Db. Later results suggest a reassignment to 256Db.
208Pb(51V,xn)259-xDb (x=1,2)
The team at Dubna also studied this reaction in 1976 and were again able to detect the 5 s SF activity, first tentatively assigned to 257Db and later to 258Db.
In 2006, the team at LBNL reinvestigated this reaction as part of their odd-Z projectile program. They were able to detect 258Db and 257Db in their measurement of the 1n and 2n neutron evaporation channels.
207Pb(51V,xn)258-xDb
The team at Dubna also studied this reaction in 1976 but this time they were unable to detect the 5 s SF activity, first tentatively assigned to 257Db and later to 258Db. Instead, they were able to measure a 1.5 s SF activity, tentatively assigned to 255Db.
205Tl(54Cr,xn)259-xDb (x=1?)
The team at Dubna also studied this reaction in 1976 and were again able to detect the 5 s SF activity, first tentatively assigned to 257Db and later to 258Db.
232Th(31P,xn)263-xDb (x=5)
There are very limited reports that this rare reaction using a P-31 beam was studied in 1989 by Andreyev et al. at the FLNR. One source suggests that no atoms were detected whilst a better source from the Russians themselves indicates that 258Db was synthesised in the 5n channel with a yield of 120 pb.
238U(27Al,xn)265-xDb (x=4,5)
In 2006, as part of their study of the use of uranium targets in superheavy element synthesis, the LBNL team led by Ken Gregorich studied the excitation functions for the 4n and 5n channels in this new reaction.
236U(27Al,xn)263-xDb (x=5,6)
This reaction was first studied by Andreyev et al. at the FLNR, Dubna in 1992. They were able to observe 258Db and 257Db in the 5n and 6n exit channels with yields of 450 pb and 75 pb, respectively.
243Am(22Ne,xn)265-xDb (x=5)
The first attempts to synthesis dubnium were performed in 1968 by the team at the Flerov Laboratory of Nuclear Reactions (FLNR) in Dubna, Russia. They observed two alpha lines which they tentatively assigned to 261Db and 260Db.
They repeated their experiment in 1970 looking for spontaneous fission
. They found a 2.2 s SF activity which they assigned to 261Db.
In 1970, the Dubna team began work on using gradient thermochromatography in order to detect dubnium in chemical experiments as a volatile chloride. In their first run they detected a volatile SF activity with similar adsorption properties to NbCl5 and unlike HfCl4. This was taken to indicate the formation of nuclei of dvi-niobium as DbCl5. In 1971, they repeated the chemistry experiment using higher sensitivity and observed alpha decays from an dvi-niobium component, taken to confirm the formation of 260105. The method was repeated in 1976 using the formation of bromides and obtained almost identical results, indicating the formation of a volatile, dvi-niobium-like [105]Br5.
241Am(22Ne,xn)263-xDb (x=4,5)
In 2000, Chinese scientists at the Institute of Modern Physics (IMP), Lanzhou, announced the discovery of the previously unknown isotope 259Db formed in the 4n neutron evaporation channel. They were also able to confirm the decay properties for 258Db.
248Cm(19F,xn)267-xDb (x=4,5)
This reaction was first studied in 1999 at the Paul Scherrer Institute (PSI) in order to produce 262Db for chemical studies. Just 4 atoms were detected with a cross section of 260 pb.
Japanese scientists at JAERI studied the reaction further in 2002 and determined yields for the isotope 262Db during their efforts to study the aqueous chemistry of dubnium.
249Bk(18O,xn)267-xDb (x=4,5)
Following from the discovery of 260Db by Albert Ghiorso in 1970 at the University of California (UC), the same team continued in 1971 with the discovery of the new isotope 262Db. They also observed an unassigned 25 s SF activity, probably associated with the now-known SF branch of 263Db.
In 1990, a team led by Kratz at LBNL definitively discovered the new isotope 263Db in the 4n neutron evaporation channel.
This reaction has been used by the same team on several occasions in order to attempt to confirm an electron capture (EC) branch in 263Db leading to long-lived 263Rf (see rutherfordium
).
249Bk(16O,xn)265-xDb (x=4)
Following from the discovery of 260Db by Albert Ghiorso in 1970 at the University of California (UC), the same team continued in 1971 with the discovery of the new isotope 261Db.
250Cf(15N,xn)265-xDb (x=4)
Following from the discovery of 260Db by Ghiorso in 1970 at LBNL, the same team continued in 1971 with the discovery of the new isotope 261Db.
249Cf(15N,xn)264-xDb (x=4)
In 1970, the team at the Lawrence Berkeley National Laboratory (LBNL) studied this reaction and identified the isotope 260Db in their discovery experiment. They used the modern technique of correlation of genetic parent-daughter decays to confirm their assignment.
In 1977, the team at Oak Ridge repeated the experiment and were able to confirm the discovery by the identification of K X-rays from the daughter lawrencium
.
254Es(13C,xn)267-xDb
In 1988, scientists as the Lawrence Livermore National Laboratory (LLNL) used the asymmetric hot fusion reaction with an einsteinium-254 target to search for the new nuclides 264Db and 263Db. Due to the low sensitivity of the experiment caused by the small Es-254 target,they were unable to detect any evaporation residues (ER).
Recent data on the decay of 272Rg has revealed that some decay chains continue through 260Db with extraordinary longer life-times than expected. These decays have been linked to an isomeric level decaying by alpha decay with a half-life of ~19 s. Further research is required to allow a definite assignment.
258Db
Evidence for an isomeric state in 258Db has been gathered from the study of the decay of 266Mt and 262Bh. It has been noted that those decays assigned to an electron capture (EC) branch has a significantly different half-life to those decaying by alpha emission. This has been taken to suggest the existence of an isomeric state decaying by EC with a half-life of ~20 s. Further experiments are required to confirm this assignment.
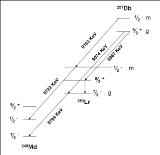
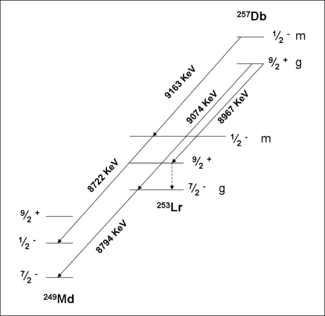
257Db
A study of the formation and decay of 257Db has proved the existence of an isomeric state. Initially, 257Db was taken to decay by alpha emission with energies 9.16,9.07 and 8.97 MeV. A measurement of the correlations of these decays with those of 253Lr have shown that the 9.16 MeV decay belongs to a separate isomer. Analysis of the data in conjunction with theory have assigned this activity to a meta stable state, 257mDb. The ground state decays by alpha emission with energies 9.07 and 8.97 MeV. Spontaneous fission of 257m,gDb was not confirmed in recent experiments.
In 1983, scientists at Dubna
carried out a series of supportive experiments in their quest for the discovery of Bohrium
. In two such experiments, they claimed they had detected a ~1.5 s spontaneous fission
activity from the reactions 207Pb(51V,xn) and 209Bi(48Ti,xn). The activity was assigned to 255Db. Later research suggested that the assignment should be changed to 256Db. As such, the isotope 255Db is currently not recognised on the chart of radionuclides and further research is required to confirm this isotope.
Niels Bohr
Niels Henrik David Bohr was a Danish physicist who made foundational contributions to understanding atomic structure and quantum mechanics, for which he received the Nobel Prize in Physics in 1922. Bohr mentored and collaborated with many of the top physicists of the century at his institute in...
. The American team proposed that the new element should be named hahnium (Ha), in honor of the late German
Germany
Germany , officially the Federal Republic of Germany , is a federal parliamentary republic in Europe. The country consists of 16 states while the capital and largest city is Berlin. Germany covers an area of 357,021 km2 and has a largely temperate seasonal climate...
chemist Otto Hahn
Otto Hahn
Otto Hahn FRS was a German chemist and Nobel laureate, a pioneer in the fields of radioactivity and radiochemistry. He is regarded as "the father of nuclear chemistry". Hahn was a courageous opposer of Jewish persecution by the Nazis and after World War II he became a passionate campaigner...
. Consequently hahnium was the name that most American and Western European scientists used and appears in many papers published at the time, and nielsbohrium was used in the Soviet Union and Eastern Bloc
Eastern bloc
The term Eastern Bloc or Communist Bloc refers to the former communist states of Eastern and Central Europe, generally the Soviet Union and the countries of the Warsaw Pact...
countries.
An element naming controversy
Element naming controversy
The names for the chemical elements 104 to 106 were the subject of a major controversy starting in the 1960s, described by some nuclear chemists as the Transfermium Wars because it concerned the elements following fermium on the periodic table....
erupted between the two groups. The International Union of Pure and Applied Chemistry
International Union of Pure and Applied Chemistry
The International Union of Pure and Applied Chemistry is an international federation of National Adhering Organizations that represents chemists in individual countries. It is a member of the International Council for Science . The international headquarters of IUPAC is located in Zürich,...
(IUPAC) thus adopted unnilpentium (Unp) as a temporary, systematic element name
Systematic element name
A systematic element name is the temporary name and symbol assigned to newly synthesized and not yet synthesized chemical elements. In chemistry, a transuranic element receives a permanent name and symbol only after its synthesis has been confirmed. In some cases, this has been a protracted and...
. Attempting to resolve the issue, in 1994, the IUPAC proposed the name joliotium (Jl), after the French physicist Frédéric Joliot-Curie
Frédéric Joliot-Curie
Jean Frédéric Joliot-Curie , born Jean Frédéric Joliot, was a French physicist and Nobel laureate.-Early years:...
, which was originally proposed by Soviet team for element 102, later named nobelium
Nobelium
Nobelium is a synthetic element with the symbol No and atomic number 102. It was first correctly identified in 1966 by scientists at the Flerov Laboratory of Nuclear Reactions in Dubna, Soviet Union...
. The two principal claimants still disagreed about the names of elements 104-106. However, in 1997 they resolved the dispute and adopted the current name, dubnium (Db), after the Russia
Russia
Russia or , officially known as both Russia and the Russian Federation , is a country in northern Eurasia. It is a federal semi-presidential republic, comprising 83 federal subjects...
n town of Dubna
Dubna
Dubna is a town in Moscow Oblast, Russia. It has a status of naukograd , being home to the Joint Institute for Nuclear Research, an international nuclear physics research centre and one of the largest scientific foundations in the country. It is also home to MKB Raduga, a defence aerospace company...
, the location of the Joint Institute for Nuclear Research
Joint Institute for Nuclear Research
The Joint Institute for Nuclear Research, JINR , in Dubna, Moscow Oblast , Russia, is an international research centre for nuclear sciences, with 5500 staff members, 1200 researchers including 1000 Ph.D.s from eighteen member states The Joint Institute for Nuclear Research, JINR , in Dubna, Moscow...
. It was argued by IUPAC that the Berkeley laboratory had already been recognized several times in the naming of elements (i.e., berkelium
Berkelium
Berkelium , is a synthetic element with the symbol Bk and atomic number 97, a member of the actinide and transuranium element series. It is named after the city of Berkeley, California, the location of the University of California Radiation Laboratory where it was discovered in December 1949...
, californium
Californium
Californium is a radioactive metallic chemical element with the symbol Cf and atomic number 98. The element was first made in the laboratory in 1950 by bombarding curium with alpha particles at the University of California, Berkeley. It is the ninth member of the actinide series and was the...
, americium
Americium
Americium is a synthetic element that has the symbol Am and atomic number 95. This transuranic element of the actinide series is located in the periodic table below the lanthanide element europium, and thus by analogy was named after another continent, America.Americium was first produced in 1944...
) and that the acceptance of the names rutherfordium
Rutherfordium
Rutherfordium is a chemical element with symbol Rf and atomic number 104, named in honor of New Zealand physicist Ernest Rutherford. It is a synthetic element and radioactive; the most stable known isotope, 267Rf, has a half-life of approximately 1.3 hours.In the periodic table of the elements,...
and seaborgium
Seaborgium
Seaborgium is a synthetic chemical element with the symbol Sg and atomic number 106.Seaborgium is a synthetic element whose most stable isotope 271Sg has a half-life of 1.9 minutes. A new isotope 269Sg has a potentially slightly longer half-life based on the observation of a single decay...
for elements 104 and 106 should be offset by recognizing the Russian team's contributions to the discovery of elements 104, 105 and 106.
Extrapolated properties
Element 105 is projected to be the second member of the 6d series of transition metals and the heaviest member of group V in the Periodic Table, below vanadiumVanadium
Vanadium is a chemical element with the symbol V and atomic number 23. It is a hard, silvery gray, ductile and malleable transition metal. The formation of an oxide layer stabilizes the metal against oxidation. The element is found only in chemically combined form in nature...
, niobium
Niobium
Niobium or columbium , is a chemical element with the symbol Nb and atomic number 41. It's a soft, grey, ductile transition metal, which is often found in the pyrochlore mineral, the main commercial source for niobium, and columbite...
and tantalum
Tantalum
Tantalum is a chemical element with the symbol Ta and atomic number 73. Previously known as tantalium, the name comes from Tantalus, a character in Greek mythology. Tantalum is a rare, hard, blue-gray, lustrous transition metal that is highly corrosion resistant. It is part of the refractory...
. Because it is positioned right below tantalum, it may also be called eka-tantalum. All the members of the group readily portray their oxidation state of +5 and the state becomes more stable as the group is descended. Thus dubnium is expected to form a stable +5 state. For this group, +4 and +3 states are also known for the heavier members and dubnium may also form these reducing oxidation states.
In an extrapolation of the chemistries from niobium
Niobium
Niobium or columbium , is a chemical element with the symbol Nb and atomic number 41. It's a soft, grey, ductile transition metal, which is often found in the pyrochlore mineral, the main commercial source for niobium, and columbite...
and tantalum
Tantalum
Tantalum is a chemical element with the symbol Ta and atomic number 73. Previously known as tantalium, the name comes from Tantalus, a character in Greek mythology. Tantalum is a rare, hard, blue-gray, lustrous transition metal that is highly corrosion resistant. It is part of the refractory...
, dubnium should react with oxygen to form an inert pentoxide, Db2O5. In alkali, the formation of an orthodubnate complex, DbO43−, is expected.
Reaction with the halogens should readily form the pentahalides, DbX5. The pentachlorides of niobium and tantalum exist as volatile solids or monomeric trigonal bipyramidal molecules in the vapour phase. Thus, DbCl5 is expected to be a volatile solid. Similarly, the pentafluoride, DbF5, should be even more volatile.
Hydrolysis of the halides is known to readily form the oxyhalides, MOX3. Thus the halides DbX5 should react with water to form DbOX3.
The reaction with fluoride ion is also well known for the lighter homologues and dubnium is expected to form a range of fluoro-complexes. In particular, reaction of the pentafluoride with HF should form a hexafluorodubnate ion, DbF6–. Excess fluoride should lead to DbF72– and DbOF52–. If eka-tantalum properties are portrayed, higher concentrations of fluoride should ultimately form DbF83– since NbF83– is not known.
Experimental chemistry
The chemistry of dubnium has been studied for several years using gas thermochromatography. The experiments have studied the relative adsorption characteristics of isotopes of niobiumNiobium
Niobium or columbium , is a chemical element with the symbol Nb and atomic number 41. It's a soft, grey, ductile transition metal, which is often found in the pyrochlore mineral, the main commercial source for niobium, and columbite...
, tantalum
Tantalum
Tantalum is a chemical element with the symbol Ta and atomic number 73. Previously known as tantalium, the name comes from Tantalus, a character in Greek mythology. Tantalum is a rare, hard, blue-gray, lustrous transition metal that is highly corrosion resistant. It is part of the refractory...
and dubnium radioisotopes. The results have indicated the formation of typical group 5 halides and oxyhalides, namely DbCl5, DbBr5, DbOCl3 and DbOBr3. Reports on these early experiments usually refer to dubnium as hahnium.
| Formula | Names(s) |
|---|---|
| DbCl5 | dubnium pentachloride ; dubnium(V) chloride |
| DbBr5 | dubnium pentabromide ; dubnium(V) bromide |
| DbOCl3 | dubnium oxychloride ; dubnium(V) trichloride oxide ; dubnyl(V) chloride |
| DbOBr3 | dubnium oxybromide ; dubnium(V) tribromide oxide ; dubnyl(V) bromide |
Cold fusion
This section deals with the synthesis of nuclei of dubnium by so-called "cold" fusion reactions. These are processes which create compound nuclei at low excitation energy (~10-20 MeV, hence "cold"), leading to a higher probability of survival from fission. The excited nucleus then decays to the ground state via the emission of one or two neutrons only.209Bi(50Ti,xn)259-xDb (x=1,2,3)
The first attempts to synthesise dubnium using cold fusion reactions were performed in 1976 by the team at FLNR, Dubna using the above reaction. They were able to detect a 5 s spontaneous fission
Spontaneous fission
Spontaneous fission is a form of radioactive decay characteristic of very heavy isotopes. Because the nuclear binding energy reaches a maximum at a nuclear mass greater than about 60 atomic mass units , spontaneous breakdown into smaller nuclei and single particles becomes possible at heavier masses...
(SF) activity which they assigned to 257Db. This assignment was later corrected to 258Db.
In 1981, the team at GSI studied this reaction using the improved technique of correlation of genetic parent-daughter decays. They were able to positively identify 258Db, the product from the 1n neutron evaporation channel.
In 1983, the team at Dubna revisited the reaction using the method of identification of a descendant using chemical separation. They succeeded in measuring alpha decays from known descendants of the decay chain beginning with 258Db. This was taken as providing some evidence for the formation of dubnium nuclei.
The team at GSI revisited the reaction in 1985 and were able to detect 10 atoms of 257Db.
After a significant upgrade of their facilities in 1993, in 2000 the team measured 120 decays of 257Db, 16 decays of 256Db and decay of 258Db in the measurement of the 1n, 2n and 3n excitation functions. The data gathered for 257Db allowed a first spectroscopic study of this isotope and identified an isomer, 257mDb, and a first determination of a decay level structure for 257Db.
The reaction was used in spectroscopic studies of isotopes of mendelevium
Mendelevium
Mendelevium is a synthetic element with the symbol Md and the atomic number 101. A metallic radioactive transuranic element in the actinide series, mendelevium is usually synthesized by bombarding einsteinium with alpha particles. It was named after Dmitri Ivanovich Mendeleev, who created the...
and einsteinium
Einsteinium
Einsteinium is a synthetic element with the symbol Es and atomic number 99. It is the seventh transuranic element, and an actinide.Einsteinium was discovered in the debris of the first hydrogen bomb explosion in 1952, and named after Albert Einstein...
in 2003-2004.
209Bi(49Ti,xn)258-xDb (x=2?)
This reaction was studied by Yuri Oganessian and the team at Dubna in 1983. They observed a 2.6 s SF activity tentatively assigned to 256Db. Later results suggest a possible reassignment to 256Rf, resulting from the ~30% EC branch in 256Db.
209Bi(48Ti,xn)257-xDb (x=1?)
This reaction was studied by Yuri Oganessian and the team at Dubna in 1983. They observed a 1.6 s activity with a ~80% alpha branch with a ~20% SF branch. The activity was tentatively assigned to 255Db. Later results suggest a reassignment to 256Db.
208Pb(51V,xn)259-xDb (x=1,2)
The team at Dubna also studied this reaction in 1976 and were again able to detect the 5 s SF activity, first tentatively assigned to 257Db and later to 258Db.
In 2006, the team at LBNL reinvestigated this reaction as part of their odd-Z projectile program. They were able to detect 258Db and 257Db in their measurement of the 1n and 2n neutron evaporation channels.
207Pb(51V,xn)258-xDb
The team at Dubna also studied this reaction in 1976 but this time they were unable to detect the 5 s SF activity, first tentatively assigned to 257Db and later to 258Db. Instead, they were able to measure a 1.5 s SF activity, tentatively assigned to 255Db.
205Tl(54Cr,xn)259-xDb (x=1?)
The team at Dubna also studied this reaction in 1976 and were again able to detect the 5 s SF activity, first tentatively assigned to 257Db and later to 258Db.
Hot fusion
This section deals with the synthesis of nuclei of dubnium by so-called "hot" fusion reactions. These are processes which create compound nuclei at high excitation energy (~40-50 MeV, hence "hot"), leading to a reduced probability of survival from fission and quasi-fission. The excited nucleus then decays to the ground state via the emission of 3-5 neutrons.232Th(31P,xn)263-xDb (x=5)
There are very limited reports that this rare reaction using a P-31 beam was studied in 1989 by Andreyev et al. at the FLNR. One source suggests that no atoms were detected whilst a better source from the Russians themselves indicates that 258Db was synthesised in the 5n channel with a yield of 120 pb.
238U(27Al,xn)265-xDb (x=4,5)
In 2006, as part of their study of the use of uranium targets in superheavy element synthesis, the LBNL team led by Ken Gregorich studied the excitation functions for the 4n and 5n channels in this new reaction.
236U(27Al,xn)263-xDb (x=5,6)
This reaction was first studied by Andreyev et al. at the FLNR, Dubna in 1992. They were able to observe 258Db and 257Db in the 5n and 6n exit channels with yields of 450 pb and 75 pb, respectively.
243Am(22Ne,xn)265-xDb (x=5)
The first attempts to synthesis dubnium were performed in 1968 by the team at the Flerov Laboratory of Nuclear Reactions (FLNR) in Dubna, Russia. They observed two alpha lines which they tentatively assigned to 261Db and 260Db.
They repeated their experiment in 1970 looking for spontaneous fission
Spontaneous fission
Spontaneous fission is a form of radioactive decay characteristic of very heavy isotopes. Because the nuclear binding energy reaches a maximum at a nuclear mass greater than about 60 atomic mass units , spontaneous breakdown into smaller nuclei and single particles becomes possible at heavier masses...
. They found a 2.2 s SF activity which they assigned to 261Db.
In 1970, the Dubna team began work on using gradient thermochromatography in order to detect dubnium in chemical experiments as a volatile chloride. In their first run they detected a volatile SF activity with similar adsorption properties to NbCl5 and unlike HfCl4. This was taken to indicate the formation of nuclei of dvi-niobium as DbCl5. In 1971, they repeated the chemistry experiment using higher sensitivity and observed alpha decays from an dvi-niobium component, taken to confirm the formation of 260105. The method was repeated in 1976 using the formation of bromides and obtained almost identical results, indicating the formation of a volatile, dvi-niobium-like [105]Br5.
241Am(22Ne,xn)263-xDb (x=4,5)
In 2000, Chinese scientists at the Institute of Modern Physics (IMP), Lanzhou, announced the discovery of the previously unknown isotope 259Db formed in the 4n neutron evaporation channel. They were also able to confirm the decay properties for 258Db.
248Cm(19F,xn)267-xDb (x=4,5)
This reaction was first studied in 1999 at the Paul Scherrer Institute (PSI) in order to produce 262Db for chemical studies. Just 4 atoms were detected with a cross section of 260 pb.
Japanese scientists at JAERI studied the reaction further in 2002 and determined yields for the isotope 262Db during their efforts to study the aqueous chemistry of dubnium.
249Bk(18O,xn)267-xDb (x=4,5)
Following from the discovery of 260Db by Albert Ghiorso in 1970 at the University of California (UC), the same team continued in 1971 with the discovery of the new isotope 262Db. They also observed an unassigned 25 s SF activity, probably associated with the now-known SF branch of 263Db.
In 1990, a team led by Kratz at LBNL definitively discovered the new isotope 263Db in the 4n neutron evaporation channel.
This reaction has been used by the same team on several occasions in order to attempt to confirm an electron capture (EC) branch in 263Db leading to long-lived 263Rf (see rutherfordium
Rutherfordium
Rutherfordium is a chemical element with symbol Rf and atomic number 104, named in honor of New Zealand physicist Ernest Rutherford. It is a synthetic element and radioactive; the most stable known isotope, 267Rf, has a half-life of approximately 1.3 hours.In the periodic table of the elements,...
).
249Bk(16O,xn)265-xDb (x=4)
Following from the discovery of 260Db by Albert Ghiorso in 1970 at the University of California (UC), the same team continued in 1971 with the discovery of the new isotope 261Db.
250Cf(15N,xn)265-xDb (x=4)
Following from the discovery of 260Db by Ghiorso in 1970 at LBNL, the same team continued in 1971 with the discovery of the new isotope 261Db.
249Cf(15N,xn)264-xDb (x=4)
In 1970, the team at the Lawrence Berkeley National Laboratory (LBNL) studied this reaction and identified the isotope 260Db in their discovery experiment. They used the modern technique of correlation of genetic parent-daughter decays to confirm their assignment.
In 1977, the team at Oak Ridge repeated the experiment and were able to confirm the discovery by the identification of K X-rays from the daughter lawrencium
Lawrencium
Lawrencium is a radioactive synthetic chemical element with the symbol Lr and atomic number 103. In the periodic table of the elements, it is a period 7 d-block element and the last element of actinide series...
.
254Es(13C,xn)267-xDb
In 1988, scientists as the Lawrence Livermore National Laboratory (LLNL) used the asymmetric hot fusion reaction with an einsteinium-254 target to search for the new nuclides 264Db and 263Db. Due to the low sensitivity of the experiment caused by the small Es-254 target,they were unable to detect any evaporation residues (ER).
Decay of heavier nuclides
Isotopes of dubnium have also been identified in the decay of heavier elements. Observations to date are summarised in the table below:| Evaporation Residue | Observed dubnium isotope |
|---|---|
| 294Uus | 270Db |
| 288Uup | 268Db |
| 287Uup | 267Db |
| 282Uut | 266Db |
| 267Bh | 263Db |
| 278Uut, 266Bh | 262Db |
| 265Bh | 261Db |
| 272Rg | 260Db |
| 266Mt, 262Bh | 258Db |
| 261Bh | 257Db |
| 260Bh | 256Db |
Isotopes
| Isotope | Year discovered | discovery reaction |
|---|---|---|
| 256Db | 1983?, 2000 | 209Bi(50Ti,3n) |
| 257Dbg | 1985 | 209Bi(50Ti,2n) |
| 257Dbm | 2000 | 209Bi(50Ti,2n) |
| 258Db | 1976?, 1981 | 209Bi(50Ti,n) |
| 259Db | 2001 | 241Am(22Ne,4n) |
| 260Db | 1970 | 249Cf(15N,4n) |
| 261Db | 1971 | 249Bk(16O,4n) |
| 262Db | 1971 | 249Bk(18O,5n) |
| 263Db | 1971?, 1990 | 249Bk(18O,4n) |
| 264Db | unknown | |
| 265Db | unknown | |
| 266Db | 2006 | 237Np(48Ca,3n) |
| 267Db | 2003 | 243Am(48CaCa,4n) |
| 268Db | 2003 | 243Am(48Ca,3n) |
| 269Db | unknown | |
| 270Db | 2009 | 249Bk(48Ca,3n) |
Isomerism
260DbRecent data on the decay of 272Rg has revealed that some decay chains continue through 260Db with extraordinary longer life-times than expected. These decays have been linked to an isomeric level decaying by alpha decay with a half-life of ~19 s. Further research is required to allow a definite assignment.
258Db
Evidence for an isomeric state in 258Db has been gathered from the study of the decay of 266Mt and 262Bh. It has been noted that those decays assigned to an electron capture (EC) branch has a significantly different half-life to those decaying by alpha emission. This has been taken to suggest the existence of an isomeric state decaying by EC with a half-life of ~20 s. Further experiments are required to confirm this assignment.
257Db
A study of the formation and decay of 257Db has proved the existence of an isomeric state. Initially, 257Db was taken to decay by alpha emission with energies 9.16,9.07 and 8.97 MeV. A measurement of the correlations of these decays with those of 253Lr have shown that the 9.16 MeV decay belongs to a separate isomer. Analysis of the data in conjunction with theory have assigned this activity to a meta stable state, 257mDb. The ground state decays by alpha emission with energies 9.07 and 8.97 MeV. Spontaneous fission of 257m,gDb was not confirmed in recent experiments.
Spectroscopic decay level schemes
257Db
Retracted isotopes
255DbIn 1983, scientists at Dubna
Dubna
Dubna is a town in Moscow Oblast, Russia. It has a status of naukograd , being home to the Joint Institute for Nuclear Research, an international nuclear physics research centre and one of the largest scientific foundations in the country. It is also home to MKB Raduga, a defence aerospace company...
carried out a series of supportive experiments in their quest for the discovery of Bohrium
Bohrium
Bohrium is a chemical element with the symbol Bh and atomic number 107 and is the heaviest member of group 7 .It is a synthetic element whose most stable known isotope, 270Bh, has a half-life of 61 seconds...
. In two such experiments, they claimed they had detected a ~1.5 s spontaneous fission
Spontaneous fission
Spontaneous fission is a form of radioactive decay characteristic of very heavy isotopes. Because the nuclear binding energy reaches a maximum at a nuclear mass greater than about 60 atomic mass units , spontaneous breakdown into smaller nuclei and single particles becomes possible at heavier masses...
activity from the reactions 207Pb(51V,xn) and 209Bi(48Ti,xn). The activity was assigned to 255Db. Later research suggested that the assignment should be changed to 256Db. As such, the isotope 255Db is currently not recognised on the chart of radionuclides and further research is required to confirm this isotope.

