Dimercaptosuccinic acid
Encyclopedia
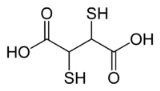
Dimercaptosuccinic acid (DMSA), is the organosulfur compound with the formula
HO2CCH(SH)CH(SH)CO2H. This colorless solid contains two carboxylic acid
and two thiol
groups, the latter being responsible for its mildly unpleasant odour. It occurs in two diastereomers, meso and the chiral dl forms. The meso isomer is used as a chelating
agent.
s), and can exist as three different stereoisomers. The 2S,3S and 2R,3R isomers are a pair of enantiomer
s, whereas the 2R,3S isomer is a meso compound
and thus optically inactive.
or fumaric
acid with sodium thiosulfate
followed by hydrolysis. The dimethyl ester
is also known.
Meso 2,3-dimercaptosuccinic acid binds to "soft"
heavy metals such as Hg2+
and Pb2+
, mobilizing these ions for excretion. It binds to metal cations through the thiol groups, which ionize upon complexation.
is 2.5-3.5 h. DMSA can cross the blood-brain barrier
of mice, but not that of humans, limiting its use to extracting heavy metals from parts of the body other than the central nervous system.
Another application for DMSA is for provocation of tissue heavy metals in anticipation of a urine test. This is sometimes called a "challenge" or "provoked" heavy metals test. DMSA is used to help mobilize heavy metals stored in body tissues (and therefore not typically present in the circulation) and increase the excretion of heavy metals in the urine. In a study by Howard Frumkin et al., this sort of test was shown to not reliably provide an indication of past chronic mercury exposure, something it was often used for. A 2004 study by GP Archbold, et al. called the results of a DMSA challenge test "misleading" for the purposes of diagnosing mercury toxicity.
Chemical formula
A chemical formula or molecular formula is a way of expressing information about the atoms that constitute a particular chemical compound....
HO2CCH(SH)CH(SH)CO2H. This colorless solid contains two carboxylic acid
Carboxylic acid
Carboxylic acids are organic acids characterized by the presence of at least one carboxyl group. The general formula of a carboxylic acid is R-COOH, where R is some monovalent functional group...
and two thiol
Thiol
In organic chemistry, a thiol is an organosulfur compound that contains a carbon-bonded sulfhydryl group...
groups, the latter being responsible for its mildly unpleasant odour. It occurs in two diastereomers, meso and the chiral dl forms. The meso isomer is used as a chelating
Chelation
Chelation is the formation or presence of two or more separate coordinate bonds between apolydentate ligand and a single central atom....
agent.
Stereochemistry
The 2,3-dimercaptosuccinic acid molecule has two stereocentres (two asymmetric carbonAsymmetric carbon
An asymmetric carbon atom is a carbon atom that is attached to four different types of atom or four different groups of atoms. Knowing the number of asymmetric carbon atoms, one can calculate the maximum possible number of stereoisomers for any given molecule as follows:As an example, malic acid...
s), and can exist as three different stereoisomers. The 2S,3S and 2R,3R isomers are a pair of enantiomer
Enantiomer
In chemistry, an enantiomer is one of two stereoisomers that are mirror images of each other that are non-superposable , much as one's left and right hands are the same except for opposite orientation. It can be clearly understood if you try to place your hands one over the other without...
s, whereas the 2R,3S isomer is a meso compound
Meso compound
A meso compound or meso isomer is a non-optically active member of a set of stereoisomers, at least two of which are optically active. This means that despite containing two or more stereocenters it is not chiral. A meso compound is superimposable on its mirror image, and it does not produce a ""...
and thus optically inactive.
| |
|
|
| |
|
|
(meso-2,3-dimercaptosuccinic acid) |
Preparation and reactivity
DMSA may be prepared by reacting maleicMaleic acid
Maleic acid is an organic compound that is a dicarboxylic acid, a molecule with two carboxyl groups. Maleic acid is the cis-isomer of butenedioic acid, whereas fumaric acid is the trans-isomer...
or fumaric
Fumaric acid
Fumaric acid or trans-butenedioic acid is the chemical compound with the formula HO2CCH=CHCO2H. This white crystalline compound is one of two isomeric unsaturated dicarboxylic acids, the other being maleic acid. In fumaric acid the carboxylic acid groups are trans and in maleic acid they are cis...
acid with sodium thiosulfate
Sodium thiosulfate
Sodium thiosulfate , also spelled sodium thiosulphate, is a colorless crystalline compound that is more familiar as the pentahydrate, Na2S2O3•5H2O, an efflorescent, monoclinic crystalline substance also called sodium hyposulfite or “hypo.”...
followed by hydrolysis. The dimethyl ester
Ester
Esters are chemical compounds derived by reacting an oxoacid with a hydroxyl compound such as an alcohol or phenol. Esters are usually derived from an inorganic acid or organic acid in which at least one -OH group is replaced by an -O-alkyl group, and most commonly from carboxylic acids and...
is also known.
Meso 2,3-dimercaptosuccinic acid binds to "soft"
HSAB theory
The HSAB concept is an acronym for 'hard and soft acids and bases. Also known as the Pearson acid base concept, HSAB is widely used in chemistry for explaining stability of compounds, reaction mechanisms and pathways....
heavy metals such as Hg2+
Mercury (element)
Mercury is a chemical element with the symbol Hg and atomic number 80. It is also known as quicksilver or hydrargyrum...
and Pb2+
Lead
Lead is a main-group element in the carbon group with the symbol Pb and atomic number 82. Lead is a soft, malleable poor metal. It is also counted as one of the heavy metals. Metallic lead has a bluish-white color after being freshly cut, but it soon tarnishes to a dull grayish color when exposed...
, mobilizing these ions for excretion. It binds to metal cations through the thiol groups, which ionize upon complexation.
Medical use
Dimercaptosuccinic acid (CHEMET) is indicated for the treatment of lead poisoning in children with blood level measured above 45 µg/dL. The use of DMSA is not approved for prophylactic/prevention of lead poisoning in anticipation of exposure in known lead contaminated environments. Its elimination half-lifeHalf-life
Half-life, abbreviated t½, is the period of time it takes for the amount of a substance undergoing decay to decrease by half. The name was originally used to describe a characteristic of unstable atoms , but it may apply to any quantity which follows a set-rate decay.The original term, dating to...
is 2.5-3.5 h. DMSA can cross the blood-brain barrier
Blood-brain barrier
The blood–brain barrier is a separation of circulating blood and the brain extracellular fluid in the central nervous system . It occurs along all capillaries and consists of tight junctions around the capillaries that do not exist in normal circulation. Endothelial cells restrict the diffusion...
of mice, but not that of humans, limiting its use to extracting heavy metals from parts of the body other than the central nervous system.
Another application for DMSA is for provocation of tissue heavy metals in anticipation of a urine test. This is sometimes called a "challenge" or "provoked" heavy metals test. DMSA is used to help mobilize heavy metals stored in body tissues (and therefore not typically present in the circulation) and increase the excretion of heavy metals in the urine. In a study by Howard Frumkin et al., this sort of test was shown to not reliably provide an indication of past chronic mercury exposure, something it was often used for. A 2004 study by GP Archbold, et al. called the results of a DMSA challenge test "misleading" for the purposes of diagnosing mercury toxicity.
See also
- Chelation therapyChelation therapyChelation therapy is the administration of chelating agents to remove heavy metals from the body. For the most common forms of heavy metal intoxication—those involving lead, arsenic or mercury—the standard of care in the United States dictates the use of dimercaptosuccinic acid...
- 2,3-Dimercapto-1-propanesulfonic acid2,3-Dimercapto-1-propanesulfonic acid2,3-Dimercapto-1-propanesulfonic acid and its sodium salt are chelating agents that form complexes with various heavy metals. They are related to dimercaprol, which is another chelating agent....
- EDTAEDTAEthylenediaminetetraacetic acid, widely abbreviated as EDTA , is a polyamino carboxylic acid and a colourless, water-soluble solid. Its conjugate base is named ethylenediaminetetraacetate. It is widely used to dissolve limescale. Its usefulness arises because of its role as a hexadentate ligand...
- Heavy metal poisoningHeavy Metal PoisoningHeavy Metal Poisoning is a song by American rock band Styx. It was included as the fifth track on their 1983 studio album Kilroy Was Here.The song in the story of Kilroy Was Here has the character of Dr Righteous preaching the evils of rock and roll...
- Mercury poisoningMercury poisoningMercury poisoning is a disease caused by exposure to mercury or its compounds. Mercury is a heavy metal occurring in several forms, all of which can produce toxic effects in high enough doses...
- Succinic acidSuccinic acidSuccinic acid is a dicarboxylic acid. Succinate plays a biochemical role in the citric acid cycle. The name derives from Latin succinum, meaning amber, from which the acid may be obtained....
- DMSA scanDMSA scanA DMSA scan is a radionucleotide scan that uses dimercaptosuccinic acid in assessing the renal function, it is now the most reliable test for the diagnosis of Acute pyelonephritis...

