
Diffusion control
Encyclopedia
Diffusion-controlled reactions
are reactions that occur so quickly that the reaction rate is the rate of transport of the reactants through the reaction medium (usually a solution). As quickly as the reactants encounter each other, they react. The process of chemical reaction can be considered as involving the diffusion of reactants until they encounter each other in the right stoichiometry and form an activated complex which can form the product species. The observed rate of chemical reactions is, generally speaking, the rate of the slowest or "rate determining" step. In diffusion controlled reactions the formation of products from the activated complex
is much faster than the diffusion of reactants and thus the rate is governed by diffusion
.
Diffusion control is rare in the gas phase, where rates of diffusion
of molecules are generally very high. Diffusion control is more likely in solution where diffusion of reactants is slower due to the greater number of collisions with solvent molecules. Reactions where the activated complex
forms easily and the products form rapidly are most likely to be limited by diffusion control. Examples are those involving catalysis
and enzymatic
reactions. Heterogeneous reactions
where reactants are in different phases are also candidates for diffusion control.
One classical test for diffusion control is to observe whether the rate of reaction is affected by stirring or agitation; if so then the reaction is almost certainly diffusion controlled under those conditions.
to estimate the upper limit of enzyme-substrate reaction
According to their estimation , the upper limit of enzyme-substrate reaction was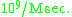
In 1972, it was observed that in the dehydration of
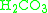 catalyzed by carbonic anhydrase, the second-order rate constant obtained experimentally was about
catalyzed by carbonic anhydrase, the second-order rate constant obtained experimentally was about
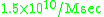 ,
,
which was one order of magnitude higher than the upper limit estimated by Alberty, Hammes, and Eigen based on a simplified model.
However, after taking into account the spatial factor and force field factor between the enzyme and its substrate, Kuo-Chen Chou and co-workers found that the upper limit could reach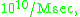
and can be used to explain some surprisingly high reaction rates in molecular biology.
The new upper limit found by Chou et al. for enzyme-substrate reaction was further confirmed by a series of follow-up studies (see, e.g.,
).
A detailed comparison between the simplified Alberty-Hammes-Eigen’s model and the Chou’s model was elaborated in the paper.
Chemical reaction
A chemical reaction is a process that leads to the transformation of one set of chemical substances to another. Chemical reactions can be either spontaneous, requiring no input of energy, or non-spontaneous, typically following the input of some type of energy, such as heat, light or electricity...
are reactions that occur so quickly that the reaction rate is the rate of transport of the reactants through the reaction medium (usually a solution). As quickly as the reactants encounter each other, they react. The process of chemical reaction can be considered as involving the diffusion of reactants until they encounter each other in the right stoichiometry and form an activated complex which can form the product species. The observed rate of chemical reactions is, generally speaking, the rate of the slowest or "rate determining" step. In diffusion controlled reactions the formation of products from the activated complex
Activated complex
In chemistry an activated complex is defined by the International Union of Pure and Applied Chemistry as "that assembly of atoms which corresponds to an arbitrary infinitesimally small region at or near the col of a potential energy surface"...
is much faster than the diffusion of reactants and thus the rate is governed by diffusion
Diffusion
Molecular diffusion, often called simply diffusion, is the thermal motion of all particles at temperatures above absolute zero. The rate of this movement is a function of temperature, viscosity of the fluid and the size of the particles...
.
Diffusion control is rare in the gas phase, where rates of diffusion
Diffusion
Molecular diffusion, often called simply diffusion, is the thermal motion of all particles at temperatures above absolute zero. The rate of this movement is a function of temperature, viscosity of the fluid and the size of the particles...
of molecules are generally very high. Diffusion control is more likely in solution where diffusion of reactants is slower due to the greater number of collisions with solvent molecules. Reactions where the activated complex
Activated complex
In chemistry an activated complex is defined by the International Union of Pure and Applied Chemistry as "that assembly of atoms which corresponds to an arbitrary infinitesimally small region at or near the col of a potential energy surface"...
forms easily and the products form rapidly are most likely to be limited by diffusion control. Examples are those involving catalysis
Catalysis
Catalysis is the change in rate of a chemical reaction due to the participation of a substance called a catalyst. Unlike other reagents that participate in the chemical reaction, a catalyst is not consumed by the reaction itself. A catalyst may participate in multiple chemical transformations....
and enzymatic
Enzyme
Enzymes are proteins that catalyze chemical reactions. In enzymatic reactions, the molecules at the beginning of the process, called substrates, are converted into different molecules, called products. Almost all chemical reactions in a biological cell need enzymes in order to occur at rates...
reactions. Heterogeneous reactions
Homogeneous and heterogeneous reactions
Homogeneity and heterogeneity are concepts relating to the uniformity or lack thereof in a substance. A material that is homogeneous is uniform in composition or character; one that is heterogeneous lacks uniformity in one of these qualities....
where reactants are in different phases are also candidates for diffusion control.
One classical test for diffusion control is to observe whether the rate of reaction is affected by stirring or agitation; if so then the reaction is almost certainly diffusion controlled under those conditions.
Applications in biology
The theory of diffusion-controlled reaction was originally utilized by R.A. Alberty, G.G. Hammes, and Manfred EigenManfred Eigen
Manfred Eigen is a German biophysical chemist who won the 1967 Nobel Prize in Chemistry for work on measuring fast chemical reactions.-Career:...
to estimate the upper limit of enzyme-substrate reaction
According to their estimation , the upper limit of enzyme-substrate reaction was
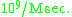
In 1972, it was observed that in the dehydration of
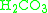 catalyzed by carbonic anhydrase, the second-order rate constant obtained experimentally was about
catalyzed by carbonic anhydrase, the second-order rate constant obtained experimentally was about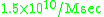 ,
,which was one order of magnitude higher than the upper limit estimated by Alberty, Hammes, and Eigen based on a simplified model.
However, after taking into account the spatial factor and force field factor between the enzyme and its substrate, Kuo-Chen Chou and co-workers found that the upper limit could reach
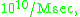
and can be used to explain some surprisingly high reaction rates in molecular biology.
The new upper limit found by Chou et al. for enzyme-substrate reaction was further confirmed by a series of follow-up studies (see, e.g.,
).
A detailed comparison between the simplified Alberty-Hammes-Eigen’s model and the Chou’s model was elaborated in the paper.

