
Dendrotoxin
Encyclopedia
Dendrotoxins are a class of neurotoxins produced by mamba
snake
s (Dendroaspis) that block particular subtypes of voltage-gated potassium channels in neurons, thereby enhancing the release of acetylcholine
at neuromuscular junctions. Because of their high potency and selectivity for potassium channels, dendrotoxins have proven to be extremely useful as pharmacological tools for studying the structure and function of these ion channel
protein
s.
the membrane during action potentials. Dendrotoxin has been shown to bind the nodes of Ranvier
of motor neurons and to block the activity of these potassium channels. In this way, dendrotoxins prolong the duration of action potentials and increase acetylcholine release at the neuromuscular junction, which may result in muscle hyperexcitability and convulsive symptoms.
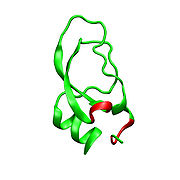 Dendrotoxins are ~7kDa proteins consisting of a single peptide chain of approximately 57-60 amino acids. Several homologues of alpha-dendrotoxin have been isolated, all possessing a slightly different sequence. However, the molecular architecture and folding conformation of these proteins are all very similar. Dendrotoxins possess a very short 310-helix near the N-terminus of the peptide, while a two turn alpha-helix
Dendrotoxins are ~7kDa proteins consisting of a single peptide chain of approximately 57-60 amino acids. Several homologues of alpha-dendrotoxin have been isolated, all possessing a slightly different sequence. However, the molecular architecture and folding conformation of these proteins are all very similar. Dendrotoxins possess a very short 310-helix near the N-terminus of the peptide, while a two turn alpha-helix
occurs near the C-terminus. A two-stranded antiparallel β-sheet
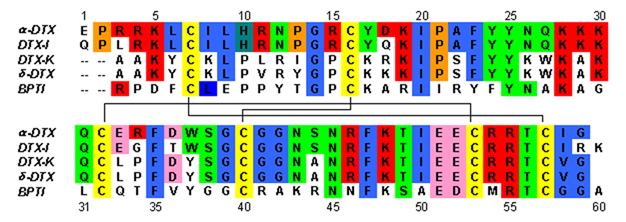
occupies the central part of the molecular structure. These two β-strands are connected by a distorted β-turn region that is thought to be important for the binding activity of the protein. All dendrotoxins are cross-linked by three disulfide bridges, which add stability to the protein and greatly contribute to its structural conformation. The cysteine
residues forming these disulfide bonds have been conserved among all members of the dendrotoxin family, and are located at C7-C57, C16-C40, and C32-C53 (numbering according to alpha-dendrotoxin).
The dendrotoxins are structurally homologous to the Kunitz-type serine protease inhibitors, including bovine pancreatic trypsin inhibitor (BPTI). Alpha-dendrotoxin and BPTI have been shown to have 35% sequence identity as well as identical disulfide bonds. Despite the structural homology between these two proteins, dendrotoxins do not appear to exhibit any measurable inhibitory protease activity like BPTI. This loss of activity appears to result from the absence of key amino acid residues that produce structural differences that hinder the key interactions necessary for the protease activity seen in BPTI.
Dendrotoxins are basic
proteins that possess a net positive charge when present in neutral pH
. Most of the positively-charged amino acid residues of dendrotoxins are located in the lower part of the structure, creating a cationic domain on one side of the protein. Positive charge results from lysine
(Lys) and arginine
(Arg) residues that are concentrated in three primary regions of the protein: near the N-terminus (Arg3, Arg4, Lys5), near the C-terminus (Arg54, Arg55) and at the narrow β-turn region (Lys28, Lys29, Lys30). It is believed that these positively-charged residues can play a critical role in dendrotoxin binding activity, as they can make potential interactions with the anionic sites (negatively-charged amino acids) in the pore of potassium channels.
to substitute positively-charged lysine and arginine residues to neutral alanine
s. These results, along with many others, have implicated that the positively-charged lysines in the N-terminal half, particularly Lys5 in the 310-helix, play a very important role in the dendrotoxin binding to their potassium channel targets. The lysine residues in the β-turn region has provided more confounding results, appearing to be biologically critical in some dendrotoxin homologues and not necessary for others. Furthermore, mutation of the entire lysine triplet (K28-K29-K30) to Ala-Ala-Gly in alpha-DTX resulted in very little change in biological activity.
There is a general agreement that the conserved lysine residue near the N-terminus (Lys5 in alpha-DTX) is crucial for the biological activity of all dendrotoxins, while additional residues, such as those in the beta-turn region, might play a role in dendrotoxin specificity by mediating the interactions of individual toxins to their individual target sites. This not only helps explain the stringent specificity of some dendrotoxins for different subtypes of voltage-gated K+ channels, but also accounts for differences in the potency of dendrotoxins for common K+ channels. For example, Wang et al. showed that the interaction of dendrotoxin-K with KV1.1 is mediated by its lysine residues in both the N-terminus and the β-turn region, while alpha-dendrotoxin appears to interact with its target solely through the N-terminus. This less expansive interactive domain may help explain why alpha-dendrotoxin is less discriminative while dendrotoxin-K is strictly selective for KV1.1.
neurons display a high degree of diversity that allows neurons to precisely tune their electrical signaling properties by expression of different combinations of potassium channel subunits. Furthermore, because they regulate ionic flux across biological membranes, they are important in many aspects of cellular regulation and signal transduction of different cell types. Therefore, voltage-gated potassium channels are targets for a wide range of potent biological toxins from such organisms as snakes, scorpion
s, sea anemones, and cone snail
s. Thus, venom purification has led to the isolation of peptide toxins such as the dendrotoxins, which have become useful pharmacological tools for the study of potassium channels. Because of their potency and selectivity for different subtypes of potassium channels, dendrotoxins have become useful as molecular probes for the structural and functional study of these proteins. This may help improve our understanding of the roles played by individual channel types, as well as assist in the pharmacological classification of these diverse channel types. Furthermore, the availability of radiolabelled dendrotoxins provides a tool for the screening of other sources in a search for new potassium channel toxins, such as the kalicludine class of potassium channel toxins in sea anemones. Lastly, the structural information provided by dendrotoxins may provide clues to the synthesis of therapeutic
compounds that may target particular classes of potassium channels.
Mamba
Mambas, of the genus Dendroaspis , are a group of highly venomous, fast-moving land-dwelling snakes of Africa. They belong to the family of Elapidae which includes cobras, coral snakes, taipans, brown snakes, tiger snakes, death adders, kraits and, debatably, sea snakes...
snake
Snake
Snakes are elongate, legless, carnivorous reptiles of the suborder Serpentes that can be distinguished from legless lizards by their lack of eyelids and external ears. Like all squamates, snakes are ectothermic, amniote vertebrates covered in overlapping scales...
s (Dendroaspis) that block particular subtypes of voltage-gated potassium channels in neurons, thereby enhancing the release of acetylcholine
Acetylcholine
The chemical compound acetylcholine is a neurotransmitter in both the peripheral nervous system and central nervous system in many organisms including humans...
at neuromuscular junctions. Because of their high potency and selectivity for potassium channels, dendrotoxins have proven to be extremely useful as pharmacological tools for studying the structure and function of these ion channel
Ion channel
Ion channels are pore-forming proteins that help establish and control the small voltage gradient across the plasma membrane of cells by allowing the flow of ions down their electrochemical gradient. They are present in the membranes that surround all biological cells...
protein
Protein
Proteins are biochemical compounds consisting of one or more polypeptides typically folded into a globular or fibrous form, facilitating a biological function. A polypeptide is a single linear polymer chain of amino acids bonded together by peptide bonds between the carboxyl and amino groups of...
s.

Functional effects in the nervous system
Dendrotoxins have been shown to block particular subtypes of voltage-gated potassium (K+) channels in neuronal tissue. In the nervous system, voltage-gated K+ channels control the excitability of nerves and muscles by controlling the resting membrane potential and by repolarizingRepolarization
In neuroscience, repolarization refers to the change in membrane potential that returns the membrane potential to a negative value after the depolarization phase of an action potential has just previously changed the membrane potential to a positive value. Repolarization results from the movement...
the membrane during action potentials. Dendrotoxin has been shown to bind the nodes of Ranvier
Nodes of Ranvier
Myelin sheath gaps or nodes of Ranvier are the gaps formed between the myelin sheaths generated by different cells. A myelin sheath is a many-layered coating, largely composed of a fatty substance called myelin, that wraps around the axon of a neuron and very efficiently insulates it...
of motor neurons and to block the activity of these potassium channels. In this way, dendrotoxins prolong the duration of action potentials and increase acetylcholine release at the neuromuscular junction, which may result in muscle hyperexcitability and convulsive symptoms.
Dendrotoxin structure

Alpha helix
A common motif in the secondary structure of proteins, the alpha helix is a right-handed coiled or spiral conformation, in which every backbone N-H group donates a hydrogen bond to the backbone C=O group of the amino acid four residues earlier...
occurs near the C-terminus. A two-stranded antiparallel β-sheet
Beta sheet
The β sheet is the second form of regular secondary structure in proteins, only somewhat less common than the alpha helix. Beta sheets consist of beta strands connected laterally by at least two or three backbone hydrogen bonds, forming a generally twisted, pleated sheet...
occupies the central part of the molecular structure. These two β-strands are connected by a distorted β-turn region that is thought to be important for the binding activity of the protein. All dendrotoxins are cross-linked by three disulfide bridges, which add stability to the protein and greatly contribute to its structural conformation. The cysteine
Cysteine
Cysteine is an α-amino acid with the chemical formula HO2CCHCH2SH. It is a non-essential amino acid, which means that it is biosynthesized in humans. Its codons are UGU and UGC. The side chain on cysteine is thiol, which is polar and thus cysteine is usually classified as a hydrophilic amino acid...
residues forming these disulfide bonds have been conserved among all members of the dendrotoxin family, and are located at C7-C57, C16-C40, and C32-C53 (numbering according to alpha-dendrotoxin).
The dendrotoxins are structurally homologous to the Kunitz-type serine protease inhibitors, including bovine pancreatic trypsin inhibitor (BPTI). Alpha-dendrotoxin and BPTI have been shown to have 35% sequence identity as well as identical disulfide bonds. Despite the structural homology between these two proteins, dendrotoxins do not appear to exhibit any measurable inhibitory protease activity like BPTI. This loss of activity appears to result from the absence of key amino acid residues that produce structural differences that hinder the key interactions necessary for the protease activity seen in BPTI.
Dendrotoxins are basic
Base (chemistry)
For the term in genetics, see base A base in chemistry is a substance that can accept hydrogen ions or more generally, donate electron pairs. A soluble base is referred to as an alkali if it contains and releases hydroxide ions quantitatively...
proteins that possess a net positive charge when present in neutral pH
PH
In chemistry, pH is a measure of the acidity or basicity of an aqueous solution. Pure water is said to be neutral, with a pH close to 7.0 at . Solutions with a pH less than 7 are said to be acidic and solutions with a pH greater than 7 are basic or alkaline...
. Most of the positively-charged amino acid residues of dendrotoxins are located in the lower part of the structure, creating a cationic domain on one side of the protein. Positive charge results from lysine
Lysine
Lysine is an α-amino acid with the chemical formula HO2CCH4NH2. It is an essential amino acid, which means that the human body cannot synthesize it. Its codons are AAA and AAG....
(Lys) and arginine
Arginine
Arginine is an α-amino acid. The L-form is one of the 20 most common natural amino acids. At the level of molecular genetics, in the structure of the messenger ribonucleic acid mRNA, CGU, CGC, CGA, CGG, AGA, and AGG, are the triplets of nucleotide bases or codons that codify for arginine during...
(Arg) residues that are concentrated in three primary regions of the protein: near the N-terminus (Arg3, Arg4, Lys5), near the C-terminus (Arg54, Arg55) and at the narrow β-turn region (Lys28, Lys29, Lys30). It is believed that these positively-charged residues can play a critical role in dendrotoxin binding activity, as they can make potential interactions with the anionic sites (negatively-charged amino acids) in the pore of potassium channels.
Mode of action
A single dendrotoxin molecule associates reversibly with a potassium channel in order to exert its inhibitory effect. It is proposed that this interaction is mediated by electrostatic interactions between the positively-charged amino acid residues in the cationic domain of dendrotoxin and the negatively-charged residues in the ion channel pore. Potassium channels, similar to other cation-selective channels, are believed to have a cloud of negative charges that precede the opening to the channel pore that help conduct potassium ions through the permeation pathway. It is generally believed (though not proven) that a dendrotoxin molecules bind to anionic sites near the extracellular surface of the channel and physically occlude the pore, thereby preventing ion conductance. However, Imredy and MacKinnon have proposed that delta-dendrotoxin may have an off-center binding site on their target proteins, and may inhibit the channel by altering the structure of the channel, rather than physically blocking the pore.Biologically important residues
Many studies have attempted to identify which amino acid residues are important for binding activity of dendrotoxins to their potassium channel targets. Harvey et al. used residue-specific modifications to identify positively-charged residues that were crucial to the blocking activity of dendrotoxin-I. They reported that acetylation of Lys5 near the N-terminal region and Lys29 in the beta-turn region led to substantial decreases in DTX-I binding affinity. Similar results have been shown with dendrotoxin-K using site-directed mutagenesisSite-directed mutagenesis
Site-directed mutagenesis, also called site-specific mutagenesis or oligonucleotide-directed mutagenesis, is a molecular biology technique in which a mutation is created at a defined site in a DNA molecule. In general, this form of mutagenesis requires that the wild type gene sequence be known...
to substitute positively-charged lysine and arginine residues to neutral alanine
Alanine
Alanine is an α-amino acid with the chemical formula CH3CHCOOH. The L-isomer is one of the 20 amino acids encoded by the genetic code. Its codons are GCU, GCC, GCA, and GCG. It is classified as a nonpolar amino acid...
s. These results, along with many others, have implicated that the positively-charged lysines in the N-terminal half, particularly Lys5 in the 310-helix, play a very important role in the dendrotoxin binding to their potassium channel targets. The lysine residues in the β-turn region has provided more confounding results, appearing to be biologically critical in some dendrotoxin homologues and not necessary for others. Furthermore, mutation of the entire lysine triplet (K28-K29-K30) to Ala-Ala-Gly in alpha-DTX resulted in very little change in biological activity.
There is a general agreement that the conserved lysine residue near the N-terminus (Lys5 in alpha-DTX) is crucial for the biological activity of all dendrotoxins, while additional residues, such as those in the beta-turn region, might play a role in dendrotoxin specificity by mediating the interactions of individual toxins to their individual target sites. This not only helps explain the stringent specificity of some dendrotoxins for different subtypes of voltage-gated K+ channels, but also accounts for differences in the potency of dendrotoxins for common K+ channels. For example, Wang et al. showed that the interaction of dendrotoxin-K with KV1.1 is mediated by its lysine residues in both the N-terminus and the β-turn region, while alpha-dendrotoxin appears to interact with its target solely through the N-terminus. This less expansive interactive domain may help explain why alpha-dendrotoxin is less discriminative while dendrotoxin-K is strictly selective for KV1.1.
Uses in research
Potassium channels of vertebrateVertebrate
Vertebrates are animals that are members of the subphylum Vertebrata . Vertebrates are the largest group of chordates, with currently about 58,000 species described. Vertebrates include the jawless fishes, bony fishes, sharks and rays, amphibians, reptiles, mammals, and birds...
neurons display a high degree of diversity that allows neurons to precisely tune their electrical signaling properties by expression of different combinations of potassium channel subunits. Furthermore, because they regulate ionic flux across biological membranes, they are important in many aspects of cellular regulation and signal transduction of different cell types. Therefore, voltage-gated potassium channels are targets for a wide range of potent biological toxins from such organisms as snakes, scorpion
Scorpion
Scorpions are predatory arthropod animals of the order Scorpiones within the class Arachnida. They have eight legs and are easily recognized by the pair of grasping claws and the narrow, segmented tail, often carried in a characteristic forward curve over the back, ending with a venomous stinger...
s, sea anemones, and cone snail
Cone snail
Conidae is a taxonomic family of minute to quite large sea snails, marine gastropod molluscs in the superfamily Conoidea.The snails within this family are sophisticated predatory animals...
s. Thus, venom purification has led to the isolation of peptide toxins such as the dendrotoxins, which have become useful pharmacological tools for the study of potassium channels. Because of their potency and selectivity for different subtypes of potassium channels, dendrotoxins have become useful as molecular probes for the structural and functional study of these proteins. This may help improve our understanding of the roles played by individual channel types, as well as assist in the pharmacological classification of these diverse channel types. Furthermore, the availability of radiolabelled dendrotoxins provides a tool for the screening of other sources in a search for new potassium channel toxins, such as the kalicludine class of potassium channel toxins in sea anemones. Lastly, the structural information provided by dendrotoxins may provide clues to the synthesis of therapeutic
Therapeutic effect
A therapeutic effect is a consequence of a medical treatment of any kind, the results of which are judged to be desirable and beneficial. This is true whether the result was expected, unexpected, or even an unintended consequence of the treatment...
compounds that may target particular classes of potassium channels.

