
Coulomb operator
Encyclopedia
The Coulomb operator, named after Charles-Augustin de Coulomb
, is a quantum mechanical
operator used in the field of quantum chemistry
. Specifically, it is a term found in the Fock operator
. It is defined as:
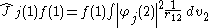
where
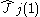 is the one-electron
is the one-electron
Coulomb operator defining the repulsion resulting from electron j,
f(1) is a one-electron wavefunction
being acted upon by the Coulomb operator,
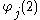 is the one-electron wavefunction of the jth electron,
is the one-electron wavefunction of the jth electron,
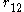 is the distance between electrons 1 and 2.
is the distance between electrons 1 and 2.
Charles-Augustin de Coulomb
Charles-Augustin de Coulomb was a French physicist. He is best known for developing Coulomb's law, the definition of the electrostatic force of attraction and repulsion. The [SI unit] of charge, the coulomb, was named after him....
, is a quantum mechanical
Quantum mechanics
Quantum mechanics, also known as quantum physics or quantum theory, is a branch of physics providing a mathematical description of much of the dual particle-like and wave-like behavior and interactions of energy and matter. It departs from classical mechanics primarily at the atomic and subatomic...
operator used in the field of quantum chemistry
Quantum chemistry
Quantum chemistry is a branch of chemistry whose primary focus is the application of quantum mechanics in physical models and experiments of chemical systems...
. Specifically, it is a term found in the Fock operator
Hartree-Fock
In computational physics and chemistry, the Hartree–Fock method is an approximate method for the determination of the ground-state wave function and ground-state energy of a quantum many-body system....
. It is defined as:
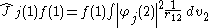
where
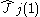 is the one-electron
is the one-electronElectron
The electron is a subatomic particle with a negative elementary electric charge. It has no known components or substructure; in other words, it is generally thought to be an elementary particle. An electron has a mass that is approximately 1/1836 that of the proton...
Coulomb operator defining the repulsion resulting from electron j,
f(1) is a one-electron wavefunction
Wavefunction
Not to be confused with the related concept of the Wave equationA wave function or wavefunction is a probability amplitude in quantum mechanics describing the quantum state of a particle and how it behaves. Typically, its values are complex numbers and, for a single particle, it is a function of...
being acted upon by the Coulomb operator,
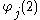 is the one-electron wavefunction of the jth electron,
is the one-electron wavefunction of the jth electron,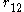 is the distance between electrons 1 and 2.
is the distance between electrons 1 and 2.

