Corey-Kim oxidation
Encyclopedia
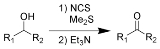
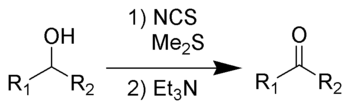
The Corey–Kim oxidation is an oxidation reaction used to synthesise aldehydes and ketones from primary and secondary alcohols. It is named for American chemist and Nobelist Elias James Corey
and Korean-American chemist Choung Un Kim.
 Although the Corey–Kim oxidation possesses the distinctive advantage over Swern oxidation
Although the Corey–Kim oxidation possesses the distinctive advantage over Swern oxidation
of allowing an operation above –25 ºC, it is not so commonly used because of the need to employ dimethyl sulfide
, a poisonous and volatile liquid with a very bad odor.
(Me2S) is treated with N-chlorosuccinimide
(NCS), resulting in formation of an "active DMSO" species that is used for the activation of the alcohol. Addition of trimethylamine
to the activated alcohol leads to its oxidation to aldehyde or ketone and generation of dimethyl sulfide. In variance with other alcohol oxidation using "activated DMSO," the reactive oxidizing species is not generated by reaction of DMSO with an electrophile. Rather, it is formed by oxidation of dimethyl sulfide with an oxidant (NCS).
Under Corey–Kim conditions allylic and benzylic alcohol
s have a tendency to evolve to the corresponding allyl and benzyl chlorides unless the alcohol activation is very quickly followed by addition of triethylamine
. In fact, Corey–Kim conditions —with no addition of triethylamine— are very efficient for the transformation of allylic and benzylic alcohols to chlorides in presence of other alcohols.
Elias James Corey
Elias James Corey is an American organic chemist. In 1990 he won the Nobel Prize in Chemistry "for his development of the theory and methodology of organic synthesis", specifically retrosynthetic analysis...
and Korean-American chemist Choung Un Kim.

Swern oxidation
The Swern oxidation, named after Daniel Swern, is a chemical reaction whereby a primary or secondary alcohol is oxidized to an aldehyde or ketone using oxalyl chloride, dimethyl sulfoxide and an organic base, such as triethylamine...
of allowing an operation above –25 ºC, it is not so commonly used because of the need to employ dimethyl sulfide
Dimethyl sulfide
Dimethyl sulfide or methylthiomethane is an organosulfur compound with the formula 2S. Dimethyl sulfide is a water-insoluble flammable liquid that boils at and has a characteristic disagreeable odor. It is a component of the smell produced from cooking of certain vegetables, notably maize,...
, a poisonous and volatile liquid with a very bad odor.
Reaction mechanism
Dimethyl sulfideDimethyl sulfide
Dimethyl sulfide or methylthiomethane is an organosulfur compound with the formula 2S. Dimethyl sulfide is a water-insoluble flammable liquid that boils at and has a characteristic disagreeable odor. It is a component of the smell produced from cooking of certain vegetables, notably maize,...
(Me2S) is treated with N-chlorosuccinimide
N-Chlorosuccinimide
N-Chlorosuccinimide is used for chlorinations and as a mild oxidant.N-Iodosuccinimide , the iodine analog of N-chlorosuccinimide, and N-bromosuccinimide , the bromine analog, are used for similar applications.-External links:...
(NCS), resulting in formation of an "active DMSO" species that is used for the activation of the alcohol. Addition of trimethylamine
Trimethylamine
Trimethylamine is an organic compound with the formula N3. This colorless, hygroscopic, and flammable tertiary amine has a strong "fishy" odor in low concentrations and an ammonia-like odor at higher concentrations...
to the activated alcohol leads to its oxidation to aldehyde or ketone and generation of dimethyl sulfide. In variance with other alcohol oxidation using "activated DMSO," the reactive oxidizing species is not generated by reaction of DMSO with an electrophile. Rather, it is formed by oxidation of dimethyl sulfide with an oxidant (NCS).
Under Corey–Kim conditions allylic and benzylic alcohol
Alcohol
In chemistry, an alcohol is an organic compound in which the hydroxy functional group is bound to a carbon atom. In particular, this carbon center should be saturated, having single bonds to three other atoms....
s have a tendency to evolve to the corresponding allyl and benzyl chlorides unless the alcohol activation is very quickly followed by addition of triethylamine
Triethylamine
Triethylamine is the chemical compound with the formula N3, commonly abbreviated Et3N. It is also abbreviated TEA, yet this abbreviation must be used carefully to avoid confusion with triethanolamine, for which TEA is also a common abbreviation....
. In fact, Corey–Kim conditions —with no addition of triethylamine— are very efficient for the transformation of allylic and benzylic alcohols to chlorides in presence of other alcohols.

