
Common Technical Document
Encyclopedia
The Common Technical Document (CTD) is a set of specification for application dossier for the registration of Medicines and designed to be used across Europe
, Japan
and the United States
. It was developed by the European Medicines Agency
(EMA, Europe
), the Food and Drug Administration
(FDA, U.S.) and the Ministry of Health, Labour and Welfare (Japan). The CTD is maintained by the International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH).
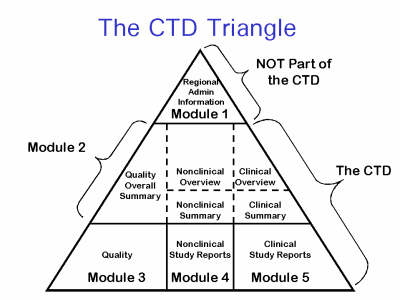
The Common Technical Document is divided into five modules:
Detailed subheadings for each Module are specified for all jurisdictions. The contents of Module 1 and certain subheadings of other Modules will differ, based on national requirements.
After the United States, European Union and Japan, the CTD has been adopted by several other countries including Canada and Switzerland.
The Paper CTD is destined to be replaced by its electronic counterpart, the eCTD
.
Europe
Europe is, by convention, one of the world's seven continents. Comprising the westernmost peninsula of Eurasia, Europe is generally 'divided' from Asia to its east by the watershed divides of the Ural and Caucasus Mountains, the Ural River, the Caspian and Black Seas, and the waterways connecting...
, Japan
Japan
Japan is an island nation in East Asia. Located in the Pacific Ocean, it lies to the east of the Sea of Japan, China, North Korea, South Korea and Russia, stretching from the Sea of Okhotsk in the north to the East China Sea and Taiwan in the south...
and the United States
United States
The United States of America is a federal constitutional republic comprising fifty states and a federal district...
. It was developed by the European Medicines Agency
European Medicines Agency
The European Medicines Agency is a European agency for the evaluation of medicinal products. From 1995 to 2004, the European Medicines Agency was known as European Agency for the Evaluation of Medicinal Products.Roughly parallel to the U.S...
(EMA, Europe
Europe
Europe is, by convention, one of the world's seven continents. Comprising the westernmost peninsula of Eurasia, Europe is generally 'divided' from Asia to its east by the watershed divides of the Ural and Caucasus Mountains, the Ural River, the Caspian and Black Seas, and the waterways connecting...
), the Food and Drug Administration
Food and Drug Administration
The Food and Drug Administration is an agency of the United States Department of Health and Human Services, one of the United States federal executive departments...
(FDA, U.S.) and the Ministry of Health, Labour and Welfare (Japan). The CTD is maintained by the International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH).

The Common Technical Document is divided into five modules:
- Administrative and prescribing information
- Overview and summary of modules 3 to 5
- Quality (pharmaceutical documentation)
- Safety (toxicology studies)
- Efficacy (clinical studies)
Detailed subheadings for each Module are specified for all jurisdictions. The contents of Module 1 and certain subheadings of other Modules will differ, based on national requirements.
After the United States, European Union and Japan, the CTD has been adopted by several other countries including Canada and Switzerland.
The Paper CTD is destined to be replaced by its electronic counterpart, the eCTD
ECTD
The electronic Common Technical Document is an interface for the pharmaceutical industry to agency transfer of regulatory information.The content is based on the Common Technical Document format....
.
See also
- Harmonization in clinical trials
- eCTDECTDThe electronic Common Technical Document is an interface for the pharmaceutical industry to agency transfer of regulatory information.The content is based on the Common Technical Document format....
- Clinical Data Interchange Standards ConsortiumClinical Data Interchange Standards ConsortiumClinical Data Interchange Standards Consortium is a non-profit organization, whose mission is "to develop and support global, platform-independent data standards that enable information system interoperability to improve medical research and related areas of health-care". Their main project, the...
- Clinical trialClinical trialClinical trials are a set of procedures in medical research and drug development that are conducted to allow safety and efficacy data to be collected for health interventions...

