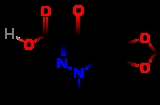
Cinoxacin
Encyclopedia
Cinoxacin has been discontinued in the U.K. as well the United States, both as a branded drug or a generic.
Cinoxacin was an older synthetic antimicrobial related to the quinolone class of antibiotics with activity similar to Oxolinic Acid and Nalidixic Acid. It was commonly used thirty years ago to treat urinary tract infections in adults. There are reports that cinoxacin had also been used to treat initial and recurrent urinary tract infections and bacterial prostatitis in dogs however this veterinary use was never approved by the United States Food and Drug Administration (FDA). In complicated UTI, the older gyrase-inhibitors such as cinoxacin are no longer indicated.
Oclassen Pharmaceuticals (Oclassen Dermatologics) commenced sales of Cinobac in the United States and Canada back in September 1992, under an agreement with Eli Lilly which granted Oclassen exclusive United States and Canadian distribution rights. Oclassen promoted Cinobac primarily to urologists for the outpatient treatment of initial and recurrent urinary tract infections and prophylaxis. Oclassen Pharmaceuticals was a privately held pharmaceutical company founded in 1985 until acquired by Watson Pharmaceuticals, Inc., in 1997. Watson Pharmaceuticals, Inc., (also incorporated in 1985), having acquired Oclassen Pharmaceuticals (Oclassen Dermatologics) also acquired the marketing rights contained within the agreement with Eli Lilly to market cinobac.
Oddly enough Watson Pharma stated in 1999 that they were marketing cinobac for the treatment of skin diseases (an unapproved use) and in 2001 that they were marketing cinobac as a general pain management product (yet another unapproved use).
It appears that this branded version was withdrawn sometime after 2003.
The CDC revoked its recommendation regarding the use of fluoroquinolones (ciprofloxacin) as a first line agent in treating anthrax (in part) due to the risk of adverse reactions documented within the Antimicrobial Postexposure Prophylaxis for Anthrax study (aka Cipro 60-day study). However, the fluoroquinolones are licensed to treat lower respiratory infections in children with cystic fibrosis in the UK. Within the United States the FDA has stated that it is their intention to pursue the licensing of the fluoroquinolones for pediatric use in spite of the evidence presented at that 62 Meeting of the Anti-Infective Drugs Advisory Committee (Circa 1997) that the fluoroquinolones cause irreversible joint damage in the pediatric population.
Note: Cinoxacin may be licensed for other uses, or restricted, by the various regulatory agencies worldwide.
, a type II topoisomerase
, and topoisomerase iv, which is an enzyme necessary to separate replicated DNA, thereby inhibiting cell division.
Evidence exists that cinoxacin binds strongly to DNA, interfering with synthesis of RNA and, consequently, with protein synthesis. The fluoroquinolones interfere with DNA replication by inhibiting an enzyme complex called DNA gyrase. This can also affect mammalian cell replication. In particular, some congeners of this drug family display high activity not only against bacterial topoisomerases but also against eukaryotic topoisomerases, and are toxic to cultured mammalian cells and in vivo tumor models. Although the quinolone is highly toxic to mammalian cells in culture, its mechanism of cytotoxic action is not known. Quinolone-induced DNA damage was first reported in 1986 (Hussy et al.).
Recent studies have demonstrated a correlation between mammalian cell cytotoxicity of the quinolones and the induction of micronuclei.
As such some fluoroquinolones may cause injury to the chromosome of eukaryotic cells.
There continues to be considerable debate as to whether or not this DNA damage is to be considered one of the mechanisms of action concerning the severe and non-abating adverse reactions experienced by some patients following fluoroquinolone therapy.
There has been a number of regulatory actions taken as a result of such adverse reactions, which included published warnings, additional warnings and safety information added to the package inserts.
In 2004 the FDA requested new warning labels to be added to all of the Fluoroquinolones, regarding Peripheral Neuropathy (irreversible nerve damage), Tendon Damage
, Heart Problems (prolonged QT Interval / Torsades de pointes), Pseudomembranous colitis
, Rhabdomyolysis (muscle wasting)
, Steven Johnson Syndrome
, as well as concurrent usage of NSAIDs contributing to the severity of these reactions. It is unknown whether or not cinobac was removed from clinical practice prior to this request by the FDA.
Children and the elderly are at a much greater risk of experiencing such adverse reactions.
Older patients may have an increased risk of tendinopathy (including rupture), especially with concomitant corticosteroid use, and such patients may also be more susceptible to prolongation of the QT interval. Patients with known prolongation, those with hypokalemia, or being treated with other drugs that prolong the QT interval should avoid the use of cinoxacin. Such reactions may manifest during, as well as long after fluoroquinolone therapy had been discontinued.
Some groups refer to these adverse events as "fluoroquinolone toxicity". These groups of people claim to have suffered serious long term harm to their health from using fluoroquinolones. This has led to a class action lawsuit by people harmed by the use of fluoroquinolones as well as legal action by the consumer advocate group Public Citizen.
Partly as a result of the efforts of The State of Illinois and Public Citizen the FDA ordered a black box warnings on all fluoroquinolones advising consumers of the possible toxic effects of fluoroquinolones on tendons.
However, unlike the other drugs found within this class, the safety profile of cinoxacin appears to be rather unremarkable. Adverse drug reactions appear to be limited to the gastrointestinal system and the central nervous system. Hypersensitivity resulting in an anaphylactic reactions (as seen with all drugs found within this class) has also been reported in association with cinoxacin. Animal studies have shown that Cinoxacin is associated with renal damage. Such damage appears to be due to the physical trauma resulting from deposition of cinoxacin crystals in the urinary tract. Such crystaluria has also been reported with other drugs in this class. A review of the literature indicates that patients treated with cinoxacin reported fewer adverse drug reactions than those treated with nalidixic acid, furadantin, amoxicillin, or trimethoprim-sulfamethoxazole.
Although phototoxicity and photoallergenicity is well demonstrated experimentally, phototoxicity does not appear to be an issue with cinoxacin. As a result of this safety profile the manufacturer, Eli Lilley states that “cinoxacin perhaps should be reserved only for those patients with organisms resistant to usual first-line agents or those who fail to respond to therapy with these agents.”
Note: Enterococcus species, Pseudomonas species, and Staphylococcus species are resistant.
. Rheumatic disease after use of a fluoroquinolone (norfloxacin
) was first reported eleven years later. In a 1995 letter published in the New England Journal of Medicine
, representatives of the U.S. Food and Drug Administration (FDA) stated that the agency would "update the labeling [package insert] for all marketed fluoroquinolones to include a warning about the possibility of tendon rupture."
By August 1996, the FDA had not taken action, and the consumer advocacy group Public Citizen
filed a petition with the FDA prompting the agency to act. Two months later, the FDA published an alert in the FDA Medical Bulletin and requested that fluoroquinolone package inserts be amended to include information on this risk.
In 2005, the Illinois Attorney General
filed a petition with the FDA seeking black box warning
s and "Dear Doctor" letters emphasizing the risk of tendon rupture; the FDA responded that it had not yet been able to reach a decision on the matter. In 2006, Public Citizen, supported by the Illinois Attorney General, renewed its demand of ten years prior for a black box warning. In January 2008, Public Citizen filed suit to compel the FDA to respond to their 2006 petition. On July 7, the FDA ordered the makers of systemic-use fluoroquinolones to add a boxed warning regarding tendon rupture, and to develop a Medication Guide for patients. The package inserts for Cipro (ciprofloxacin
), Avelox (moxifloxacin
), Proquin XR, Factive (gemifloxacin
), Floxin (ofloxacin
), Noroxin (norfloxacin
) and Levaquin (levofloxacin) were amended on September 8, 2008 to include these new warnings. Bayer
, which manufactures Cipro, Avelox and Proquin XR, issued a Dear Healthcare Professional letter on October 22 concerning these changes. Ortho-McNeil, the manufacturers of Levaquin, issued a similar letter in November. through the Health Care Notification Network, a registration-only website that distributes drug alerts to licensed healthcare professionals.
Review of the FDA website indicates that the majority of the generic versions of the fluoroquinolones have not been updated to include this Black Box Warning as of July 2009. And there are numerous reports that this information has not been dessiminated to the pharmacist, the products continue to contain the previous labels that are absent of this warning, and the Medication Guide has not been made available to the pharmicist or physician for distribution.
These adverse drug reactions are easily and likely often misdiagnosed as seizure disorder or regular CNS or psychiatric symptoms and the diagnosis of quinolone toxicity or adverse reaction missed. Research conducted in Italy has shown that quinolones such as cinoxacin are one of the top causes of CNS disturbances in general practice. Another Italian study done in 2008 showed a far greater risk of an adverse reaction occurring with cinoxacin (cinoxacin was related to the highest ROR value) as compared to the other quinolone drugs.
Increased hospitalizations attributed to adverse drug reactions alone account for billions of dollars each year within the US healthcare system. Severe reactions do occur with the fluoroquinolone class and can add significantly to the cost of care. Antibacterial adverse effects account for nearly 25% of all adverse drug reactions amongst hospitalized patients. “Indirect costs as a result of reduced quality of life or loss of productivity are certainly not reflected in the acquisition costs of antimicrobials.”
The adverse drug reaction profile of cinoxacin and other fluoroquinolone drugs has spawned a grass root movement of those so affected to lobby for Black Box Warnings and Dear Doctor Letters as well as the petitioning of the FDA for the removal of some fluoroquinolone drugs from clinical practice.
Cinoxacin was an older synthetic antimicrobial related to the quinolone class of antibiotics with activity similar to Oxolinic Acid and Nalidixic Acid. It was commonly used thirty years ago to treat urinary tract infections in adults. There are reports that cinoxacin had also been used to treat initial and recurrent urinary tract infections and bacterial prostatitis in dogs however this veterinary use was never approved by the United States Food and Drug Administration (FDA). In complicated UTI, the older gyrase-inhibitors such as cinoxacin are no longer indicated.
History
Cinoxacin is one of the original quinolone drugs, which were introduced in the 1970s. Commonly referred to as the first generation quinolones. This first generation also included other quinolone drugs such as pipemidic acid, and oxolinic acid, but this first generation proved to be only marginal improvements over nalidixic acid. Cinoxacin is similar chemically (and in antimicrobial activity) to oxolonic acid and nalidixic acid. Relative to nalidixic acid, cinoxacin was found to have a slightly greater inhibitory and bactericidal activity. Cinoxacin was patented in 1972 and assigned to Eli Lilly. Eli Lilly obtained approval from the FDA to market cinoxacin in the United States as Cinobac on June 13, 1980. Prior to this cinobac was marketed in the U.K. and Switzerland in 1979.Oclassen Pharmaceuticals (Oclassen Dermatologics) commenced sales of Cinobac in the United States and Canada back in September 1992, under an agreement with Eli Lilly which granted Oclassen exclusive United States and Canadian distribution rights. Oclassen promoted Cinobac primarily to urologists for the outpatient treatment of initial and recurrent urinary tract infections and prophylaxis. Oclassen Pharmaceuticals was a privately held pharmaceutical company founded in 1985 until acquired by Watson Pharmaceuticals, Inc., in 1997. Watson Pharmaceuticals, Inc., (also incorporated in 1985), having acquired Oclassen Pharmaceuticals (Oclassen Dermatologics) also acquired the marketing rights contained within the agreement with Eli Lilly to market cinobac.
Oddly enough Watson Pharma stated in 1999 that they were marketing cinobac for the treatment of skin diseases (an unapproved use) and in 2001 that they were marketing cinobac as a general pain management product (yet another unapproved use).
It appears that this branded version was withdrawn sometime after 2003.
Pediatric restrictions
Cinobac is not approved to treat pediatric patients. Prescribing Cinobac to treat an unapproved use (other than Urinary tract infections) within the pediatric, as well as the adult population, does take place rather frequently. The fluoroquinolones are not licensed by the FDA for use in children (other than the exception inhalational anthrax, which is restricted to levaquin and ciprofloxacin) due to the risk of fatalities as well as permanent injury to the musculoskeletal system. Although alleged to be effective, neither ciprofloxacin or levofloxacin is considered to be a first line agent for inhalational anthrax in the pediatric population due to severe adverse reactions involving the musculoskeletal system and other serious adverse reactions, including fatalities.The CDC revoked its recommendation regarding the use of fluoroquinolones (ciprofloxacin) as a first line agent in treating anthrax (in part) due to the risk of adverse reactions documented within the Antimicrobial Postexposure Prophylaxis for Anthrax study (aka Cipro 60-day study). However, the fluoroquinolones are licensed to treat lower respiratory infections in children with cystic fibrosis in the UK. Within the United States the FDA has stated that it is their intention to pursue the licensing of the fluoroquinolones for pediatric use in spite of the evidence presented at that 62 Meeting of the Anti-Infective Drugs Advisory Committee (Circa 1997) that the fluoroquinolones cause irreversible joint damage in the pediatric population.
Note: Cinoxacin may be licensed for other uses, or restricted, by the various regulatory agencies worldwide.
Mode of action
Cinoxacin mode of action involves the inhibiting of DNA gyraseDNA gyrase
DNA gyrase, often referred to simply as gyrase, is an enzyme that relieves strain while double-stranded DNA is being unwound by helicase. This causes negative supercoiling of the DNA...
, a type II topoisomerase
Topoisomerase
Topoisomerases are enzymes that regulate the overwinding or underwinding of DNA. The winding problem of DNA arises due to the intertwined nature of its double helical structure. For example, during DNA replication, DNA becomes overwound ahead of a replication fork...
, and topoisomerase iv, which is an enzyme necessary to separate replicated DNA, thereby inhibiting cell division.
Evidence exists that cinoxacin binds strongly to DNA, interfering with synthesis of RNA and, consequently, with protein synthesis. The fluoroquinolones interfere with DNA replication by inhibiting an enzyme complex called DNA gyrase. This can also affect mammalian cell replication. In particular, some congeners of this drug family display high activity not only against bacterial topoisomerases but also against eukaryotic topoisomerases, and are toxic to cultured mammalian cells and in vivo tumor models. Although the quinolone is highly toxic to mammalian cells in culture, its mechanism of cytotoxic action is not known. Quinolone-induced DNA damage was first reported in 1986 (Hussy et al.).
Recent studies have demonstrated a correlation between mammalian cell cytotoxicity of the quinolones and the induction of micronuclei.
As such some fluoroquinolones may cause injury to the chromosome of eukaryotic cells.
There continues to be considerable debate as to whether or not this DNA damage is to be considered one of the mechanisms of action concerning the severe and non-abating adverse reactions experienced by some patients following fluoroquinolone therapy.
Contraindications
Within the most recent package insert (circa 1999) Cinobac is listed as being contraindicated in patients with a history of hypersensitivity to cinoxacin or other quinolones.Adverse reactions
Serious adverse events occur more commonly with fluoroquinolones than with any other antibiotic drug classes.There has been a number of regulatory actions taken as a result of such adverse reactions, which included published warnings, additional warnings and safety information added to the package inserts.
In 2004 the FDA requested new warning labels to be added to all of the Fluoroquinolones, regarding Peripheral Neuropathy (irreversible nerve damage), Tendon Damage
, Heart Problems (prolonged QT Interval / Torsades de pointes), Pseudomembranous colitis
, Rhabdomyolysis (muscle wasting)
, Steven Johnson Syndrome
, as well as concurrent usage of NSAIDs contributing to the severity of these reactions. It is unknown whether or not cinobac was removed from clinical practice prior to this request by the FDA.
Children and the elderly are at a much greater risk of experiencing such adverse reactions.
Older patients may have an increased risk of tendinopathy (including rupture), especially with concomitant corticosteroid use, and such patients may also be more susceptible to prolongation of the QT interval. Patients with known prolongation, those with hypokalemia, or being treated with other drugs that prolong the QT interval should avoid the use of cinoxacin. Such reactions may manifest during, as well as long after fluoroquinolone therapy had been discontinued.
Some groups refer to these adverse events as "fluoroquinolone toxicity". These groups of people claim to have suffered serious long term harm to their health from using fluoroquinolones. This has led to a class action lawsuit by people harmed by the use of fluoroquinolones as well as legal action by the consumer advocate group Public Citizen.
Partly as a result of the efforts of The State of Illinois and Public Citizen the FDA ordered a black box warnings on all fluoroquinolones advising consumers of the possible toxic effects of fluoroquinolones on tendons.
However, unlike the other drugs found within this class, the safety profile of cinoxacin appears to be rather unremarkable. Adverse drug reactions appear to be limited to the gastrointestinal system and the central nervous system. Hypersensitivity resulting in an anaphylactic reactions (as seen with all drugs found within this class) has also been reported in association with cinoxacin. Animal studies have shown that Cinoxacin is associated with renal damage. Such damage appears to be due to the physical trauma resulting from deposition of cinoxacin crystals in the urinary tract. Such crystaluria has also been reported with other drugs in this class. A review of the literature indicates that patients treated with cinoxacin reported fewer adverse drug reactions than those treated with nalidixic acid, furadantin, amoxicillin, or trimethoprim-sulfamethoxazole.
Although phototoxicity and photoallergenicity is well demonstrated experimentally, phototoxicity does not appear to be an issue with cinoxacin. As a result of this safety profile the manufacturer, Eli Lilley states that “cinoxacin perhaps should be reserved only for those patients with organisms resistant to usual first-line agents or those who fail to respond to therapy with these agents.”
Overdose
Symptoms following an overdose of cinoxacin may include anorexia, nausea, vomiting, epigastric distress, and diarrhea. The severity of the epigastric distress and the diarrhea are dose related. Patients who have ingested an overdose of cinoxacin should be kept well hydrated to prevent crystalluria. Forced diuresis, peritoneal dialysis, hemodialysis, or charcoal hemoperfusion have not been established as beneficial for an overdose of cinoxacin.Pharmacokinetics
Biotransformation is mainly hepatic, with approximately 30-40% metabolized to inactive metabolites. Protein Binding ranges from 60 to 80%. Cinoxacin is rapidly absorbed after oral administration. The presence of food delays absorption but does not affect total absorption. The mean serum half-life is 1.5 hours. Half-life in patients with impaired renal function may exceed 10 hours.Dosing
The usual adult dosage for the treatment of urinary tract infections is 1 g daily, administered orally in 2 or 4 divided doses (500 mg b.i.d. or 250 mg q.i.d. respectively) for 7 to 14 days.Susceptible bacteria
Gram-negative aerobes:- Enterobacter species
- Escherichia coli
- Klebsiella species
- Proteus mirabilis
- Proteus vulgaris
Note: Enterococcus species, Pseudomonas species, and Staphylococcus species are resistant.
Regulatory history
The regulatory history in the United States regarding cinoxacin has been deleted from the FDA site. Though approved in 1980, there is only one document (circa 2002) available for reference. Twenty two years worth or regulatory history concerning cinoxacin is unavailable.History of the black box warnings
Musculoskeletal disorders attributed to use of quinolone antibiotics were first reported in the medical literature in 1972, as an adverse reaction to nalidixic acidNalidixic acid
Nalidixic acid is the first of the synthetic quinolone antibiotics...
. Rheumatic disease after use of a fluoroquinolone (norfloxacin
Norfloxacin
Norfloxacin is a synthetic chemotherapeutic antibacterial agent occasionally used to treat common as well as complicated urinary tract infections. It is sold under various brand names with the most common being Noroxin. In form of ophthalmic solutions it is known as Chibroxin...
) was first reported eleven years later. In a 1995 letter published in the New England Journal of Medicine
New England Journal of Medicine
The New England Journal of Medicine is an English-language peer-reviewed medical journal published by the Massachusetts Medical Society. It describes itself as the oldest continuously published medical journal in the world.-History:...
, representatives of the U.S. Food and Drug Administration (FDA) stated that the agency would "update the labeling [package insert] for all marketed fluoroquinolones to include a warning about the possibility of tendon rupture."
By August 1996, the FDA had not taken action, and the consumer advocacy group Public Citizen
Public Citizen
Public Citizen is a non-profit, consumer rights advocacy group based in Washington, D.C., United States, with a branch in Austin, Texas. Public Citizen was founded by Ralph Nader in 1971, headed for 26 years by Joan Claybrook, and is now headed by Robert Weissman.-Lobbying Efforts:Public Citizen...
filed a petition with the FDA prompting the agency to act. Two months later, the FDA published an alert in the FDA Medical Bulletin and requested that fluoroquinolone package inserts be amended to include information on this risk.
In 2005, the Illinois Attorney General
Illinois Attorney General
The Illinois Attorney General is the highest legal officer of the state of Illinois in the United States. Originally an appointed office, it is now an office filled by election through universal suffrage...
filed a petition with the FDA seeking black box warning
Black box warning
In the United States, a black box warning is a type of warning that appears on the package insert for prescription drugs that may cause serious adverse effects...
s and "Dear Doctor" letters emphasizing the risk of tendon rupture; the FDA responded that it had not yet been able to reach a decision on the matter. In 2006, Public Citizen, supported by the Illinois Attorney General, renewed its demand of ten years prior for a black box warning. In January 2008, Public Citizen filed suit to compel the FDA to respond to their 2006 petition. On July 7, the FDA ordered the makers of systemic-use fluoroquinolones to add a boxed warning regarding tendon rupture, and to develop a Medication Guide for patients. The package inserts for Cipro (ciprofloxacin
Ciprofloxacin
Ciprofloxacin is a synthetic chemotherapeutic antibiotic of the fluoroquinolone drug class.It is a second-generation fluoroquinolone antibacterial. It kills bacteria by interfering with the enzymes that cause DNA to rewind after being copied, which stops synthesis of DNA and of...
), Avelox (moxifloxacin
Moxifloxacin
Moxifloxacin is a fourth-generation synthetic fluoroquinolone antibacterial agent developed by Bayer AG . It is marketed worldwide under the brand names Avelox, Avalox, and Avelon for oral treatment. In most countries, the drug is also available in parenteral form for intravenous infusion...
), Proquin XR, Factive (gemifloxacin
Gemifloxacin
Gemifloxacin mesylate is an oral broad-spectrum quinolone antibacterial agent used in the treatment of acute bacterial exacerbation of chronic bronchitis and mild-to-moderate pneumonia....
), Floxin (ofloxacin
Ofloxacin
Ofloxacin is a synthetic chemotherapeutic antibiotic of the fluoroquinolone drug class considered to be a second-generation fluoroquinolone. The original brand, Floxin, has been discontinued by the manufacturer in the United States on 18 June 2009, though generic equivalents continue to be...
), Noroxin (norfloxacin
Norfloxacin
Norfloxacin is a synthetic chemotherapeutic antibacterial agent occasionally used to treat common as well as complicated urinary tract infections. It is sold under various brand names with the most common being Noroxin. In form of ophthalmic solutions it is known as Chibroxin...
) and Levaquin (levofloxacin) were amended on September 8, 2008 to include these new warnings. Bayer
Bayer
Bayer AG is a chemical and pharmaceutical company founded in Barmen , Germany in 1863. It is headquartered in Leverkusen, North Rhine-Westphalia, Germany and well known for its original brand of aspirin.-History:...
, which manufactures Cipro, Avelox and Proquin XR, issued a Dear Healthcare Professional letter on October 22 concerning these changes. Ortho-McNeil, the manufacturers of Levaquin, issued a similar letter in November. through the Health Care Notification Network, a registration-only website that distributes drug alerts to licensed healthcare professionals.
Review of the FDA website indicates that the majority of the generic versions of the fluoroquinolones have not been updated to include this Black Box Warning as of July 2009. And there are numerous reports that this information has not been dessiminated to the pharmacist, the products continue to contain the previous labels that are absent of this warning, and the Medication Guide has not been made available to the pharmicist or physician for distribution.
Social and economic impact
Spontaneous reports of adverse drug reactions associated with cinoxacin, as well as other drugs found within the fluoroquinolone class, are repeatedly made on many Internet forums and medication feedback sites related to prescription drugs worldwide.These adverse drug reactions are easily and likely often misdiagnosed as seizure disorder or regular CNS or psychiatric symptoms and the diagnosis of quinolone toxicity or adverse reaction missed. Research conducted in Italy has shown that quinolones such as cinoxacin are one of the top causes of CNS disturbances in general practice. Another Italian study done in 2008 showed a far greater risk of an adverse reaction occurring with cinoxacin (cinoxacin was related to the highest ROR value) as compared to the other quinolone drugs.
Increased hospitalizations attributed to adverse drug reactions alone account for billions of dollars each year within the US healthcare system. Severe reactions do occur with the fluoroquinolone class and can add significantly to the cost of care. Antibacterial adverse effects account for nearly 25% of all adverse drug reactions amongst hospitalized patients. “Indirect costs as a result of reduced quality of life or loss of productivity are certainly not reflected in the acquisition costs of antimicrobials.”
The adverse drug reaction profile of cinoxacin and other fluoroquinolone drugs has spawned a grass root movement of those so affected to lobby for Black Box Warnings and Dear Doctor Letters as well as the petitioning of the FDA for the removal of some fluoroquinolone drugs from clinical practice.

