
Chemical transport reaction
Encyclopedia
In chemistry
, a chemical transport reaction describes a process for purification and crystallization
of non-volatile
solid
s. The process is also responsible for certain aspects of mineral growth from the effluent of volcano
es. The technique is distinct from chemical vapor deposition
, which usually entails decomposition of molecular precursors (e.g. SiH4 → Si + 2H2) and which gives conformal coatings.
The technique, which was popularized by Schäfer, entails the reversible conversion of nonvolatile element
s and chemical compound
s into volatile derivatives. The volatile derivative migrates throughout a sealed reactor, typically a sealed, evacuated glass tube heated in a tube furnace
. Because the tube is under a temperature gradient, the volatile
derivative reverts to the parent solid and the transport agent is released at the end opposite to which it originated (see next section). The transport agent is thus catalytic. The technique requires that the two ends of the tube (which contains the sample to be crystallized) be maintained at different temperatures. So-called two-zone tube furnaces are employed for this purpose. The method derives from the Van Arkel de Boer process
which was used for the purification of titanium and vanadium and uses iodine as the transport agent.

, then the solid of interest is transported from the cooler end (which can be quite hot) of the reactor to a hot end, where the equilibrium constant is less favorable and the crystals grow. The reaction of molybdenum dioxide
with the transporting agent iodine
is an exothermic process, thus the MoO2 migrates from the cooler end (700 °C) to the hotter end (900 °C):
Using 10 milligrams of iodine for 4 grams of the solid, the process requires several days.
Alternatively, when the reaction of the solid and the transport agent is endothermic, the solid is transported from a hot zone to a cooler one. For example:
The sample of iron(III) oxide is maintained at 1000 °C, and the product is grown at 750 °C. HCl is the transport agent. Crystals of hematite
are reportedly observed at the mouths of volcanoes because of chemical transport reactions whereby volcanic hydrogen chloride volatilizes iron(III) oxides.
s. The tungsten is evaporated from the tungsten filament and converted with traces of oxygen and iodine into the WO2I2, at the high temperatures near the filament the compound decomposes back to tungsten oxygen and iodine.
Chemistry
Chemistry is the science of matter, especially its chemical reactions, but also its composition, structure and properties. Chemistry is concerned with atoms and their interactions with other atoms, and particularly with the properties of chemical bonds....
, a chemical transport reaction describes a process for purification and crystallization
Crystallization
Crystallization is the process of formation of solid crystals precipitating from a solution, melt or more rarely deposited directly from a gas. Crystallization is also a chemical solid–liquid separation technique, in which mass transfer of a solute from the liquid solution to a pure solid...
of non-volatile
Volatility (chemistry)
In chemistry and physics, volatility is the tendency of a substance to vaporize. Volatility is directly related to a substance's vapor pressure. At a given temperature, a substance with higher vapor pressure vaporizes more readily than a substance with a lower vapor pressure.The term is primarily...
solid
Solid
Solid is one of the three classical states of matter . It is characterized by structural rigidity and resistance to changes of shape or volume. Unlike a liquid, a solid object does not flow to take on the shape of its container, nor does it expand to fill the entire volume available to it like a...
s. The process is also responsible for certain aspects of mineral growth from the effluent of volcano
Volcano
2. Bedrock3. Conduit 4. Base5. Sill6. Dike7. Layers of ash emitted by the volcano8. Flank| 9. Layers of lava emitted by the volcano10. Throat11. Parasitic cone12. Lava flow13. Vent14. Crater15...
es. The technique is distinct from chemical vapor deposition
Chemical vapor deposition
Chemical vapor deposition is a chemical process used to produce high-purity, high-performance solid materials. The process is often used in the semiconductor industry to produce thin films. In a typical CVD process, the wafer is exposed to one or more volatile precursors, which react and/or...
, which usually entails decomposition of molecular precursors (e.g. SiH4 → Si + 2H2) and which gives conformal coatings.
The technique, which was popularized by Schäfer, entails the reversible conversion of nonvolatile element
Chemical element
A chemical element is a pure chemical substance consisting of one type of atom distinguished by its atomic number, which is the number of protons in its nucleus. Familiar examples of elements include carbon, oxygen, aluminum, iron, copper, gold, mercury, and lead.As of November 2011, 118 elements...
s and chemical compound
Chemical compound
A chemical compound is a pure chemical substance consisting of two or more different chemical elements that can be separated into simpler substances by chemical reactions. Chemical compounds have a unique and defined chemical structure; they consist of a fixed ratio of atoms that are held together...
s into volatile derivatives. The volatile derivative migrates throughout a sealed reactor, typically a sealed, evacuated glass tube heated in a tube furnace
Tube furnace
A tube furnace is an electric heating device used to conduct syntheses and purifications of inorganic compounds and occasionally in organic synthesis. The usual design consists of a cylindrical cavity surrounded by heating coils, which are embedded in a thermally insulating matrix. Temperature is...
. Because the tube is under a temperature gradient, the volatile
Volatility (chemistry)
In chemistry and physics, volatility is the tendency of a substance to vaporize. Volatility is directly related to a substance's vapor pressure. At a given temperature, a substance with higher vapor pressure vaporizes more readily than a substance with a lower vapor pressure.The term is primarily...
derivative reverts to the parent solid and the transport agent is released at the end opposite to which it originated (see next section). The transport agent is thus catalytic. The technique requires that the two ends of the tube (which contains the sample to be crystallized) be maintained at different temperatures. So-called two-zone tube furnaces are employed for this purpose. The method derives from the Van Arkel de Boer process
Crystal bar process
The crystal bar process was developed by Anton Eduard van Arkel and Jan Hendrik de Boer in 1925. This process was the first industrial process for the commercial production of pure ductile metallic zirconium. It is used in the production of small quantities of ultra-pure titanium and zirconium...
which was used for the purification of titanium and vanadium and uses iodine as the transport agent.

Cases of the exothermic and endothermic reactions of the transporting agent
Transport reactions are classified according to the thermodynamics of the reaction between the solid and the transporting agent. When the reaction is exothermicExothermic
In thermodynamics, the term exothermic describes a process or reaction that releases energy from the system, usually in the form of heat, but also in the form of light , electricity , or sound...
, then the solid of interest is transported from the cooler end (which can be quite hot) of the reactor to a hot end, where the equilibrium constant is less favorable and the crystals grow. The reaction of molybdenum dioxide
Molybdenum dioxide
Molybdenum dioxide is the chemical compound with the formula MoO2. It is a violet-colored solid and is a metallic conductor. It crystallizes in a monoclinic cell, and has a distorted rutile, crystal structure. In TiO2 the oxide anions are close packed and titanium atoms occupy half of the...
with the transporting agent iodine
Iodine
Iodine is a chemical element with the symbol I and atomic number 53. The name is pronounced , , or . The name is from the , meaning violet or purple, due to the color of elemental iodine vapor....
is an exothermic process, thus the MoO2 migrates from the cooler end (700 °C) to the hotter end (900 °C):
- MoO2 + I2 MoO2I2 ΔHrxn < 0 (exothermic)
Using 10 milligrams of iodine for 4 grams of the solid, the process requires several days.
Alternatively, when the reaction of the solid and the transport agent is endothermic, the solid is transported from a hot zone to a cooler one. For example:
- Fe2O3Iron(III) oxideIron oxide or ferric oxide is the inorganic compound with the formula Fe2O3. It is one of the three main oxides of iron, the other two being iron oxide , which is rare, and iron oxide , which also occurs naturally as the mineral magnetite. As the mineral known as hematite, Fe2O3 is the main...
+ 6 HClHydrogen chlorideThe compound hydrogen chloride has the formula HCl. At room temperature, it is a colorless gas, which forms white fumes of hydrochloric acid upon contact with atmospheric humidity. Hydrogen chloride gas and hydrochloric acid are important in technology and industry...
Fe2Cl6Iron(III) chlorideIron chloride, also called ferric chloride, is an industrial scale commodity chemical compound, with the formula FeCl3. The colour of iron chloride crystals depends on the viewing angle: by reflected light the crystals appear dark green, but by transmitted light they appear purple-red...
+ 3 H2O ΔHrxn > 0 (endothermic)
The sample of iron(III) oxide is maintained at 1000 °C, and the product is grown at 750 °C. HCl is the transport agent. Crystals of hematite
Hematite
Hematite, also spelled as haematite, is the mineral form of iron oxide , one of several iron oxides. Hematite crystallizes in the rhombohedral system, and it has the same crystal structure as ilmenite and corundum...
are reportedly observed at the mouths of volcanoes because of chemical transport reactions whereby volcanic hydrogen chloride volatilizes iron(III) oxides.
Halogen lamp
A similar reaction like that of MoO2 is used in halogen lampHalogen lamp
A halogen lamp, also known as a tungsten halogen lamp, is an incandescent lamp with a tungsten filament contained within an inert gas and a small amount of a halogen such as iodine or bromine. The chemical halogen cycle redeposits evaporated tungsten back on to the filament, extending the life of...
s. The tungsten is evaporated from the tungsten filament and converted with traces of oxygen and iodine into the WO2I2, at the high temperatures near the filament the compound decomposes back to tungsten oxygen and iodine.
- WO2 + I2
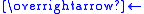 WO2I2 ΔHrxn < 0 (exothermic)
WO2I2 ΔHrxn < 0 (exothermic)

