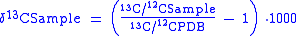Carbon-13
Encyclopedia
Carbon-13 is a natural, stable
isotope of carbon
and one of the environmental isotopes
. It makes up about 1.1% of all natural carbon on Earth
.
of an organic compound will usually contain a small peak of one mass unit greater than the apparent molecular ion peak (M). This is known as the M+1 peak and originates due to the presence of 13C atoms. A molecule containing one carbon atom will be expected to have an M+1 peak of approximately 1.1% of the size of the M peak as 1.1% of the carbon atoms will be 13C rather than 12C
. Similarly a molecule containing two carbon atoms will be expected to have an M+1 peak of approximately 2.2% of the size of the M peak, as there is double the previous likelihood that a molecule will contain a 13C atom.
In the above the mathematics and chemistry have been simplified, however it can be used effectively to give the number of carbon atoms for small to medium sized organic molecules. In the following formula the result should be rounded to the nearest integer
:
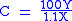
C = number of C atoms X = amplitude
of the M ion peak Y = amplitude of the M+1 ion peak
13C-enriched compounds are used in the research of metabolic processes by means of mass spectrometry. Such compounds are safe because they are non-radioactive. In addition, 13C is used to quantify proteins (quantitative proteomics
). One important application is in "Stable isotope labeling with amino acids in cell culture" (SILAC
). 13C-enriched compounds are used in medical diagnostic tests such as the Urea breath test
. Analysis in these tests is usually of the ratio of 13-C to 12-C by Isotope ratio mass spectrometry
The ratio of 13C to 12C is slightly higher in plants employing C4 carbon fixation
than in plants employing C3 carbon fixation
. Because the different isotope ratios for the two kinds of plants propagate through the food chain, it is possible to determine if the principal diet of a human or other animal consists primarily of C3 plants or C4 plants by measuring the isotopic signature
of their collagen and other tissues. Deliberate increase of proportion of 13C in diet is the concept of i-food
, a proposed way to increase longevity
.
To calculate δ13C:
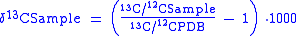
Stable isotope
Stable isotopes are chemical isotopes that may or may not be radioactive, but if radioactive, have half-lives too long to be measured.Only 90 nuclides from the first 40 elements are energetically stable to any kind of decay save proton decay, in theory...
isotope of carbon
Carbon
Carbon is the chemical element with symbol C and atomic number 6. As a member of group 14 on the periodic table, it is nonmetallic and tetravalent—making four electrons available to form covalent chemical bonds...
and one of the environmental isotopes
Environmental isotopes
The environmental isotopes are a subset of the isotopes, both stable and radioactive, which are the object of Isotope geochemistry.The most used environmental isotopes are:* deuterium* tritium* carbon-13* carbon-14* nitrogen-15* oxygen-18...
. It makes up about 1.1% of all natural carbon on Earth
Earth
Earth is the third planet from the Sun, and the densest and fifth-largest of the eight planets in the Solar System. It is also the largest of the Solar System's four terrestrial planets...
.
Detection by mass spectrometry
A mass spectrumMass spectrum
A mass spectrum is an intensity vs. m/z plot representing a chemical analysis. Hence, the mass spectrum of a sample is a pattern representing the distribution of ions by mass in a sample. It is a histogram usually acquired using an instrument called a mass spectrometer...
of an organic compound will usually contain a small peak of one mass unit greater than the apparent molecular ion peak (M). This is known as the M+1 peak and originates due to the presence of 13C atoms. A molecule containing one carbon atom will be expected to have an M+1 peak of approximately 1.1% of the size of the M peak as 1.1% of the carbon atoms will be 13C rather than 12C
Carbon-12
Carbon-12 is the more abundant of the two stable isotopes of the element carbon, accounting for 98.89% of carbon; it contains 6 protons, 6 neutrons, and 6 electrons....
. Similarly a molecule containing two carbon atoms will be expected to have an M+1 peak of approximately 2.2% of the size of the M peak, as there is double the previous likelihood that a molecule will contain a 13C atom.
In the above the mathematics and chemistry have been simplified, however it can be used effectively to give the number of carbon atoms for small to medium sized organic molecules. In the following formula the result should be rounded to the nearest integer
Integer
The integers are formed by the natural numbers together with the negatives of the non-zero natural numbers .They are known as Positive and Negative Integers respectively...
:
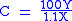
C = number of C atoms X = amplitude
Amplitude
Amplitude is the magnitude of change in the oscillating variable with each oscillation within an oscillating system. For example, sound waves in air are oscillations in atmospheric pressure and their amplitudes are proportional to the change in pressure during one oscillation...
of the M ion peak Y = amplitude of the M+1 ion peak
13C-enriched compounds are used in the research of metabolic processes by means of mass spectrometry. Such compounds are safe because they are non-radioactive. In addition, 13C is used to quantify proteins (quantitative proteomics
Proteomics
Proteomics is the large-scale study of proteins, particularly their structures and functions. Proteins are vital parts of living organisms, as they are the main components of the physiological metabolic pathways of cells. The term "proteomics" was first coined in 1997 to make an analogy with...
). One important application is in "Stable isotope labeling with amino acids in cell culture" (SILAC
Silac
SILAC is a technique based on mass spectrometry that detects differences in protein abundance among samples using non-radioactive isotopic labeling. It is a popular method for quantitative proteomics.-Procedure:Two populations of cells are cultivated in cell culture...
). 13C-enriched compounds are used in medical diagnostic tests such as the Urea breath test
Urea breath test
The urea breath test is a rapid diagnostic procedure used to identify infections by Helicobacter pylori, a spiral bacterium implicated in gastritis, gastric ulcer, and peptic ulcer disease. It is based upon the ability of H. pylori to convert urea to ammonia and carbon dioxide...
. Analysis in these tests is usually of the ratio of 13-C to 12-C by Isotope ratio mass spectrometry
The ratio of 13C to 12C is slightly higher in plants employing C4 carbon fixation
C4 carbon fixation
C4 carbon fixation is one of three biochemical mechanisms, along with and CAM photosynthesis, used in carbon fixation. It is named for the 4-carbon molecule present in the first product of carbon fixation in these plants, in contrast to the 3-carbon molecule products in plants. fixation is an...
than in plants employing C3 carbon fixation
C3 carbon fixation
carbon fixation is a metabolic pathway for carbon fixation in photosynthesis. This process converts carbon dioxide and ribulose bisphosphate into 3-phosphoglycerate through the following reaction:...
. Because the different isotope ratios for the two kinds of plants propagate through the food chain, it is possible to determine if the principal diet of a human or other animal consists primarily of C3 plants or C4 plants by measuring the isotopic signature
Isotopic signature
An isotopic signature is a ratio of stable or unstable isotopes of particular elements found in an investigated material...
of their collagen and other tissues. Deliberate increase of proportion of 13C in diet is the concept of i-food
I-food
iFood contains nutritients in which some atoms are replaced with their heavier non-radioactive isotopes . Biomolecules that incorporate heavier isotopes give rise to more stable molecular structures with increased resistance to damages associated with ageing...
, a proposed way to increase longevity
Longevity
The word "longevity" is sometimes used as a synonym for "life expectancy" in demography or known as "long life", especially when it concerns someone or something lasting longer than expected ....
.
Uses in earth science
Due to differential uptake in plants as well as marine carbonates of 13C, it is possible to use these isotopic signature in earth science. In aqueous geochemistry, by analyzing the δ13C value of surface and ground waters the source of the water can be identified. This is due to the fact that atmospheric, carbonate, and plant derived δ13C values all differ with respect to Pee Dee Belemnite (PDB) standard.To calculate δ13C: