Bromocresol green
Encyclopedia
Bromocresol Green is a dye of the triphenylmethane
family (triarylmethane dye
s), which is used as a pH indicator
and as a tracking dye for DNA
agarose gel electrophoresis. It can be used in its free acid form (light brown solid), or as a sodium salt (dark green solid). It is also an inhibitor of the prostaglandin E2 transport protein.
In aqueous solution
, both solids ionize
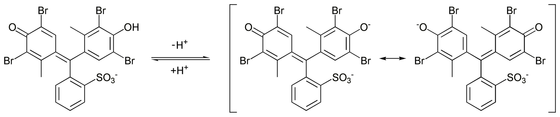
to give the monoanionic form (yellow), that further deprotonates at higher pH to give the dianionic form (blue), which is stabilized by resonance
:
 The pK
The pK
(pKa
) of this reaction is 4.8.
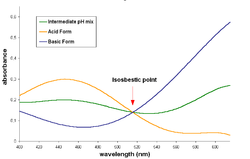
The acid and basic form of this dye have an isosbestic point
in their spectra
, around 515 nm.
 Ethanol solution (0.04 wt.%) of Bromocresol Green has been proposed for TLC staining and is suitable for visualisation of the compounds with functional groups whose pKa is below 5.0 (carboxylic acids, sulfonic acids etc.). These appear as yellow spots on light or dark blue background; no heating is necessary. Bromophenol Blue
Ethanol solution (0.04 wt.%) of Bromocresol Green has been proposed for TLC staining and is suitable for visualisation of the compounds with functional groups whose pKa is below 5.0 (carboxylic acids, sulfonic acids etc.). These appear as yellow spots on light or dark blue background; no heating is necessary. Bromophenol Blue
solution can be used for the same purpose.
The compound is synthesized by bromination of Cresol Purple (m-cresolsulfonphthalein).
Triphenylmethane
Triphenylmethane, or triphenyl methane, is the hydrocarbon with the formula 3CH. This colorless solid is soluble in nonpolar organic solvents and not in water. Triphenylmethane has the basic skeleton of many synthetic dyes called triarylmethane dyes, many of them are pH indicators, and some display...
family (triarylmethane dye
Triarylmethane dye
Triarylmethane dyes are synthetic organic compounds containing triphenylmethane backbones. As dyes, these compounds are intensely colored. Many of these dyes undergo reactions in response to acid and base, and thus serve as pH indicators....
s), which is used as a pH indicator
PH indicator
A pH indicator is a halochromic chemical compound that is added in small amounts to a solution so that the pH of the solution can be determined visually. Hence a pH indicator is a chemical detector for hydronium ions or hydrogen ions in the Arrhenius model. Normally, the indicator causes the...
and as a tracking dye for DNA
DNA
Deoxyribonucleic acid is a nucleic acid that contains the genetic instructions used in the development and functioning of all known living organisms . The DNA segments that carry this genetic information are called genes, but other DNA sequences have structural purposes, or are involved in...
agarose gel electrophoresis. It can be used in its free acid form (light brown solid), or as a sodium salt (dark green solid). It is also an inhibitor of the prostaglandin E2 transport protein.
In aqueous solution
Aqueous solution
An aqueous solution is a solution in which the solvent is water. It is usually shown in chemical equations by appending aq to the relevant formula, such as NaCl. The word aqueous means pertaining to, related to, similar to, or dissolved in water...
, both solids ionize
Dissociation (chemistry)
Dissociation in chemistry and biochemistry is a general process in which ionic compounds separate or split into smaller particles, ions, or radicals, usually in a reversible manner...
to give the monoanionic form (yellow), that further deprotonates at higher pH to give the dianionic form (blue), which is stabilized by resonance
Resonance (chemistry)
In chemistry, resonance or mesomerism is a way of describing delocalized electrons within certain molecules or polyatomic ions where the bonding cannot be expressed by one single Lewis formula...
:

Dissociation constant
In chemistry, biochemistry, and pharmacology, a dissociation constant is a specific type of equilibrium constant that measures the propensity of a larger object to separate reversibly into smaller components, as when a complex falls apart into its component molecules, or when a salt splits up into...
(pKa
PKA
PKA, pKa, or other similar variations may stand for:* pKa, the symbol for the acid dissociation constant at logarithmic scale* Protein kinase A, a class of cAMP-dependent enzymes* Pi Kappa Alpha, the North-American social fraternity...
) of this reaction is 4.8.
The acid and basic form of this dye have an isosbestic point
Isosbestic point
In spectroscopy, an isosbestic point is a specific wavelength at which two chemical species have the same molar absorptivity or -more generally- are linearly related...
in their spectra
Electromagnetic spectrum
The electromagnetic spectrum is the range of all possible frequencies of electromagnetic radiation. The "electromagnetic spectrum" of an object is the characteristic distribution of electromagnetic radiation emitted or absorbed by that particular object....
, around 515 nm.

Bromophenol blue
Bromophenol blue is used as an acid-base indicator, a color marker and a dye.-Acid-base indicator:As an acid-base indicator its useful range lies between pH 3.0 and 4.6...
solution can be used for the same purpose.
The compound is synthesized by bromination of Cresol Purple (m-cresolsulfonphthalein).

