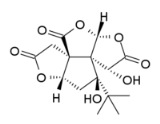
Bilobalide
Encyclopedia
Bilobalide is a biologically active terpenic
trilactone
present in Ginkgo biloba.
is still unknown.
(GGPP), which is formed by addition of farnesyl pyrophosphate
(FPP) to isopentenyl pyrophosphate
(IPP) unit, to form a C15 sesquiterpene
. Such formation went through the mevalonate pathway (MVA) and methylerythritol phosphate MEP pathway. In order to generate bilobalide, C20 ginkgolide 13 must form first. To transform from GGPP to abietenyl cation 5, a single bifunctional enzyme abietadiene synthase
E1 is required. However, due to the complexity of ginkgolide structures for rearrangement, ring cleavage, and formation of lactone rings, diterpene 8 is used to explain instead. Levopimaradiene 6 and abietatriene 7 are precursors for ginkgolide and bilobalide formation. The unusual tert-butyl substituent is formed from A ring cleavage in 9. Bilobalide 13 then formed in loss of carbons through degradation from ginkgolide 12, and lactones are formed from residual carboxyl and alcohol functions. The end product of bilobalide contains sesquiterpenes and three lactones units.
at the GABAA and GABAA-rho receptors. Of GABAA, it may possibly be selective for the subunits predominantly implicated in cognitive and memory functioning such as α1
.
Terpenoid
The terpenoids , sometimes called isoprenoids, are a large and diverse class of naturally occurring organic chemicals similar to terpenes, derived from five-carbon isoprene units assembled and modified in thousands of ways. Most are multicyclic structures that differ from one another not only in...
trilactone
Lactone
In chemistry, a lactone is a cyclic ester which can be seen as the condensation product of an alcohol group -OH and a carboxylic acid group -COOH in the same molecule...
present in Ginkgo biloba.
Chemistry
Bilobalide is a main constituent of the terpenoids found in Ginkgo leaves. It also exists in minor amounts in the roots. It is a sesquiterpenoid, i.e. it has a 15-carbon skeleton. Its exact synthesis pathway from farnesyl pyrophosphateFarnesyl pyrophosphate
Farnesyl pyrophosphate is an intermediate in the HMG-CoA reductase pathway used by organisms in the biosynthesis of terpenes, terpenoids, and sterols...
is still unknown.
Biosynthesis
Bilobalide and ginkgolide both have very similar biosynthesis pathway. Bilobalide is formed by partially degraded from ginkgoglide. Bilibalide is derived from geranylgeranyl pyrophosphateGeranylgeranyl pyrophosphate
Geranylgeranyl pyrophosphate is an intermediate in the HMG-CoA reductase pathway used by organisms in the biosynthesis of terpenes and terpenoids. In plants it is also the precursor to carotenoids, gibberellins, tocopherols, and chlorophylls....
(GGPP), which is formed by addition of farnesyl pyrophosphate
Farnesyl pyrophosphate
Farnesyl pyrophosphate is an intermediate in the HMG-CoA reductase pathway used by organisms in the biosynthesis of terpenes, terpenoids, and sterols...
(FPP) to isopentenyl pyrophosphate
Isopentenyl pyrophosphate
Isopentenyl pyrophosphate is an intermediate in the classical, HMG-CoA reductase pathway used by organisms in the biosynthesis of terpenes and terpenoids. IPP is formed from acetyl-CoA via mevalonic acid...
(IPP) unit, to form a C15 sesquiterpene
Sesquiterpene
Sesquiterpenes are a class of terpenes that consist of three isoprene units and have the molecular formula C15H24. Like monoterpenes, sesquiterpenes may be acyclic or contain rings, including many unique combinations...
. Such formation went through the mevalonate pathway (MVA) and methylerythritol phosphate MEP pathway. In order to generate bilobalide, C20 ginkgolide 13 must form first. To transform from GGPP to abietenyl cation 5, a single bifunctional enzyme abietadiene synthase
Abietadiene synthase
In enzymology, an abietadiene synthase is an enzyme that catalyzes the chemical reaction-copalyl diphosphate \rightleftharpoons -abietadiene + diphosphate...
E1 is required. However, due to the complexity of ginkgolide structures for rearrangement, ring cleavage, and formation of lactone rings, diterpene 8 is used to explain instead. Levopimaradiene 6 and abietatriene 7 are precursors for ginkgolide and bilobalide formation. The unusual tert-butyl substituent is formed from A ring cleavage in 9. Bilobalide 13 then formed in loss of carbons through degradation from ginkgolide 12, and lactones are formed from residual carboxyl and alcohol functions. The end product of bilobalide contains sesquiterpenes and three lactones units.
Pharmacology
Bilobalide is important for producing several of the effects of Gingko biloba extracts, and it has neuroprotective effects, as well as inducing the liver enzymes CYP3A1 and 1A2, which may be partially responsible for interactions between gingko and other herbal medicines or pharmaceutical drugs. Bilobalide has recently been found to be an antagonistReceptor antagonist
A receptor antagonist is a type of receptor ligand or drug that does not provoke a biological response itself upon binding to a receptor, but blocks or dampens agonist-mediated responses...
at the GABAA and GABAA-rho receptors. Of GABAA, it may possibly be selective for the subunits predominantly implicated in cognitive and memory functioning such as α1
GABRA1
Gamma-aminobutyric acid receptor subunit alpha-1 is a protein that in humans is encoded by the GABRA1 gene.-Further reading:...
.

