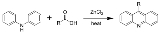
Bernthsen acridine synthesis
Encyclopedia
The Bernthsen acridine synthesis is the chemical reaction
of a diarylamine heated with a carboxylic acid
(or acid anhydride) and zinc chloride
to form a 9-substituted acridine
.
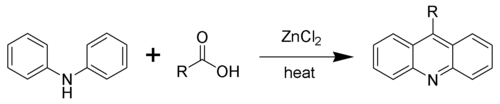 Using zinc chloride, one must heat the reaction to 200-270 °C for 24hrs. The use of polyphosphoric acid will give acridine products at a lower temperature, but also with decreased yields.
Using zinc chloride, one must heat the reaction to 200-270 °C for 24hrs. The use of polyphosphoric acid will give acridine products at a lower temperature, but also with decreased yields.
Chemical reaction
A chemical reaction is a process that leads to the transformation of one set of chemical substances to another. Chemical reactions can be either spontaneous, requiring no input of energy, or non-spontaneous, typically following the input of some type of energy, such as heat, light or electricity...
of a diarylamine heated with a carboxylic acid
Carboxylic acid
Carboxylic acids are organic acids characterized by the presence of at least one carboxyl group. The general formula of a carboxylic acid is R-COOH, where R is some monovalent functional group...
(or acid anhydride) and zinc chloride
Zinc chloride
Zinc chloride is the name of chemical compound with the formula ZnCl2 and its hydrates. Zinc chlorides, of which nine crystalline forms are known, are colorless or white, and are highly soluble in water. ZnCl2 itself is hygroscopic and even deliquescent. Samples should therefore be protected from...
to form a 9-substituted acridine
Acridine
Acridine, C13H9N, is an organic compound and a nitrogen heterocycle. Acridine is also used to describe compounds containing the C13N tricycle....
.


