
Argon Oxygen Decarburization
Encyclopedia
Argon oxygen decarburization (AOD) is a process primarily used in stainless steel
making and other high grade alloys with oxidizable elements such as chromium
and aluminum. After initial melting the metal is then transferred to an AOD vessel where it will be subjected to three steps of refining; decarburization
, reduction, and desulphurization. AOD was invented in 1954 by the Lindé Division of The Union Carbide Corporation (which became known as Praxair
in 1992) .
The decarburization step is controlled by ratios of oxygen to argon or nitrogen to remove the carbon from the metal bath. The ratios can be done in any number of phases to facilitate the reaction. The gases are usually blown through a top lance (oxygen only) and tuyeres in the sides/bottom (oxygen with an inert gas shroud). The stages of blowing remove carbon by the combination of oxygen and carbon forming CO gas.
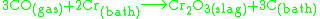
To drive the reaction to the forming of CO the partial pressure of CO is lowered using argon or nitrogen. Since the AOD vessel isn't externally heated, the blowing stages are also used for temperature control. The burning of oxygen increases the bath temperature.

So, additions of lime are added to dilute sulfur in the metal bath. Also, aluminum or silicon may be added to remove oxygen. Other trimming alloy additions might be added at the end of the step. After sulfur levels have been achieved the slag is removed from the AOD vessel and the metal bath is ready for tapping. The tapped bath is then either sent to a stir station for further chemistry trimming or to a caster for casting.
Stainless steel
In metallurgy, stainless steel, also known as inox steel or inox from French "inoxydable", is defined as a steel alloy with a minimum of 10.5 or 11% chromium content by mass....
making and other high grade alloys with oxidizable elements such as chromium
Chromium
Chromium is a chemical element which has the symbol Cr and atomic number 24. It is the first element in Group 6. It is a steely-gray, lustrous, hard metal that takes a high polish and has a high melting point. It is also odorless, tasteless, and malleable...
and aluminum. After initial melting the metal is then transferred to an AOD vessel where it will be subjected to three steps of refining; decarburization
Decarburization
Decarburization is the process opposite to carburization, namely aimed at decreasing the content of carbon in metals . Decarburization occurs when Carbon in the metal reacts with gasses present in the atmosphere...
, reduction, and desulphurization. AOD was invented in 1954 by the Lindé Division of The Union Carbide Corporation (which became known as Praxair
Praxair
Praxair, Inc. is the largest industrial gases company in North and South America and one of the largest worldwide. The company supplies atmospheric, process and specialty gases as well as high-performance coatings and related services to a wide diversity of customers around the world...
in 1992) .
Decarburization
Prior to the decarburization step, one more step should be taken into consideration: de-siliconization, which is very important factor for refractory lining and further processing.The decarburization step is controlled by ratios of oxygen to argon or nitrogen to remove the carbon from the metal bath. The ratios can be done in any number of phases to facilitate the reaction. The gases are usually blown through a top lance (oxygen only) and tuyeres in the sides/bottom (oxygen with an inert gas shroud). The stages of blowing remove carbon by the combination of oxygen and carbon forming CO gas.
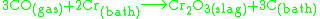
To drive the reaction to the forming of CO the partial pressure of CO is lowered using argon or nitrogen. Since the AOD vessel isn't externally heated, the blowing stages are also used for temperature control. The burning of oxygen increases the bath temperature.
Reduction
After a desired carbon and temperature level have been reached the process moves to reduction. Reduction recovers the oxidized elements such as chromium from the slag. To achieve this, alloy additions are made with elements that have a higher affinity for oxygen than chromium, using either a Silicon alloy or Aluminum. The reduction mix also includes lime (CaO) and fluorspar (CaF2). The addition of lime and fluorspar help with driving the reduction of Cr2O3 and managing the slag, keeping the slag fluid and volume small.Desulphurization
Desulphurization is achieved by having a high lime concentration in the slag and a low oxygen activity in the metal bath.
So, additions of lime are added to dilute sulfur in the metal bath. Also, aluminum or silicon may be added to remove oxygen. Other trimming alloy additions might be added at the end of the step. After sulfur levels have been achieved the slag is removed from the AOD vessel and the metal bath is ready for tapping. The tapped bath is then either sent to a stir station for further chemistry trimming or to a caster for casting.

