
Anodic stripping voltammetry
Encyclopedia
Analyte
An analyte, or component , is a substance or chemical constituent that is of interest in an analytical procedure. Grammatically, it is important to note that experiments always seek to measure properties of analytes—and that analytes themselves can never be measured. For instance, one cannot...
of interest is electroplated
Electroplating
Electroplating is a plating process in which metal ions in a solution are moved by an electric field to coat an electrode. The process uses electrical current to reduce cations of a desired material from a solution and coat a conductive object with a thin layer of the material, such as a metal...
on the working electrode
Working electrode
The working electrode is the electrode in an electrochemical system on which the reaction of interest is occurring. The working electrode is often used in conjunction with an auxiliary electrode, and a reference electrode in a three electrode system...
during a deposition step, and oxidized from the electrode during the stripping step. The current is measured during the stripping step. The oxidation of species is registered as a peak in the current signal at the potential at which the species begins to be oxidized. The stripping step can be either linear
Linear sweep voltammetry
Linear sweep voltammetry is a voltammetric method where the current at a working electrode is measured while the potential between the working electrode and a reference electrode is swept linearly in time...
, staircase
Staircase voltammetry
Staircase voltammetry is a derivative of linear sweep voltammetry. In linear sweep voltammetry the current at a working electrode is measured while the potential between the working electrode and a reference electrode is swept linearly in time...
, squarewave
Squarewave voltammetry
Squarewave voltammetry is a further improvement of staircase voltammetry which is itself a derivative of linear sweep voltammetry. In linear sweep voltammetry the current at a working electrode is measured while the potential between the working electrode and a reference electrode is swept...
, or pulse.
Electrochemical Cell Set-Up
Anodic stripping voltammetry usually incorporates three electrodes, a working electrodeWorking electrode
The working electrode is the electrode in an electrochemical system on which the reaction of interest is occurring. The working electrode is often used in conjunction with an auxiliary electrode, and a reference electrode in a three electrode system...
, auxiliary electrode
Auxiliary electrode
The Auxiliary electrode, often also called the counter electrode, is an electrode used in a three electrode electrochemical cell for voltammetric analysis or other reactions in which an electrical current is expected to flow...
(sometimes called the counter electrode), and reference electrode
Reference electrode
A reference electrode is an electrode which has a stable and well-known electrode potential. The high stability of the electrode potential is usually reached by employing a redox system with constant concentrations of each participants of the redox reaction.There are many ways reference...
. The solution
Solution
In chemistry, a solution is a homogeneous mixture composed of only one phase. In such a mixture, a solute is dissolved in another substance, known as a solvent. The solvent does the dissolving.- Types of solutions :...
being analyzed usually has an electrolyte
Electrolyte
In chemistry, an electrolyte is any substance containing free ions that make the substance electrically conductive. The most typical electrolyte is an ionic solution, but molten electrolytes and solid electrolytes are also possible....
added to it. For most standard tests, the working electrode is a mercury
Mercury (element)
Mercury is a chemical element with the symbol Hg and atomic number 80. It is also known as quicksilver or hydrargyrum...
film electrode. The mercury film forms an amalgam
Amalgam (chemistry)
An amalgam is a substance formed by the reaction of mercury with another metal. Almost all metals can form amalgams with mercury, notable exceptions being iron and platinum. Silver-mercury amalgams are important in dentistry, and gold-mercury amalgam is used in the extraction of gold from ore.The...
with the analyte of interest, which upon oxidation results in a sharp peak, improving resolution between analytes. The mercury film is formed over a glassy carbon
Glassy carbon
Glassy carbon, also called vitreous carbon, is a non-graphitizing carbon which combines glassy and ceramic properties with those of graphite. The most important properties are high temperature resistance, hardness , low density, low electrical resistance, low friction, low thermal resistance,...
electrode. A mercury drop electrode has also been used for much the same reasons. In cases where the analyte of interest has an oxidizing potential above that of mercury, or where a mercury electrode would be otherwise unsuitable, a solid, inert metal such as silver
Silver
Silver is a metallic chemical element with the chemical symbol Ag and atomic number 47. A soft, white, lustrous transition metal, it has the highest electrical conductivity of any element and the highest thermal conductivity of any metal...
, gold
Gold
Gold is a chemical element with the symbol Au and an atomic number of 79. Gold is a dense, soft, shiny, malleable and ductile metal. Pure gold has a bright yellow color and luster traditionally considered attractive, which it maintains without oxidizing in air or water. Chemically, gold is a...
, or platinum
Platinum
Platinum is a chemical element with the chemical symbol Pt and an atomic number of 78. Its name is derived from the Spanish term platina del Pinto, which is literally translated into "little silver of the Pinto River." It is a dense, malleable, ductile, precious, gray-white transition metal...
may also be used.
Steps
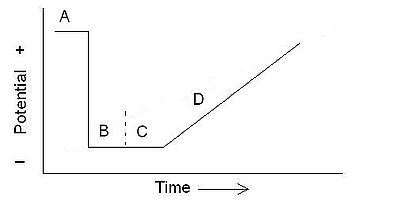
Anodic stripping voltammetry usually incorporates 4 steps if the working electrode is a mercury film or mercury drop electrode and the solution incorporates stirring. The solution is stirred during the first two steps at a repeatable rate. The first step is a cleaning step; in the cleaning step, the potential is held at a more oxidizing potential than the analyte of interest for a period of time in order to fully remove it from the electrode. In the second step, the potential is held at a lower potential, low enough to reduce the analyte and deposit it on the electrode. After the second step, the stirring is stopped, and the electrode is kept at the lower potential. The purpose of this third step is to allow the deposited material to distribute more evenly in the mercury. If a solid inert electrode is used, this step is unnecessary. The last step involves raising the working electrode to a higher potential (anodic), and stripping (oxidizing) the analyte. As the analyte is oxidized, it gives off electrons which are measured as a current.Stripping analysis is an analytical technique that involves (i) preconcentration of a metal phase onto a solid electrode surface or into Hg (liquid) at negative potentials and (ii) selective oxidation of each metal phase species during an anodic potential sweep.
Stripping analysis has the following properties:
- Very sensitive and reproducible (RSD<5%) method for trace metal ion analysis in aqueous media.
- Concentration limits of detection for many metals are in the low ppb to high pptrange (S/N=3) and this compares favorably with AAS or ICP analysis.
- Field deployable instrumentation that is inexpensive.
- Approximately 12-15 metal ions can be analyzed for by this method.
- The stripping peak currents and peak widths are a function of the size, coverage and distribution of the metal phase on the electrode surface (Hg or alternate)
Sensitivity
Anodic stripping voltammetry can detect μg/l concentrations of analyte. This method has an excellent detection limit (typically 10−9 - 10−10 M)See also
- Cathodic stripping voltammetryCathodic stripping voltammetryCathodic stripping voltammetry is a voltammetric method for quantitative determination of specific ionic species. It is similar to the trace analysis method anodic stripping voltammetry, except that for the plating step, the potential is held at an oxidizing potential, and the oxidized species are...
- Adsorptive stripping voltammetryAdsorptive stripping voltammetryAdsorpive stripping voltammetry is similar to anodic stripping voltammetry and cathodic stripping voltammetry except that the preconcentration step is not controlled by electrolysis. The preconcentration step in adsorptive stripping voltammetry is accomplished by adsorption on the working...
- VoltammetryVoltammetryVoltammetry is a category of electroanalytical methods used in analytical chemistry and various industrial processes. In voltammetry, information about an analyte is obtained by measuring the current as the potential is varied.- Three electrode system :...
- Electroanalytical Methods
- ElectroplatingElectroplatingElectroplating is a plating process in which metal ions in a solution are moved by an electric field to coat an electrode. The process uses electrical current to reduce cations of a desired material from a solution and coat a conductive object with a thin layer of the material, such as a metal...

