
Alfred Wohl
Encyclopedia
Alfred Wohl 3 October 1863 – 25 December 1939) was a German
chemist
.
Several chemical reactions are named after him, including the Wohl degradation
, Wohl-Aue reaction
and the Wohl-Ziegler reaction
.
. He became an assistant of Hermann Emil Fischer
at the University of Berlin from 1886 until 1891, where he also received his habilitation
. He became professor at the University of Berlin in 1901, but he left for the Technical University of Danzig in 1904. He retired because of antisemitic pressure in 1933, but worked in his lab until 1937. He emigrated to Sweden in 1938, where he died in 1939.
in 1886. Under the influence of Fischer, Wohl focused on sugar chemistry. Wohl arbitrarily defined the structure of (+)-glyceraldehyde to have the D-configuration, forming the basis for the D-L system. This was done before chemists had the ability to prove (+)-glyceraldehyde was, in fact, D-glyceraldehyde. Before the invention of x-ray crystallography
the exact determination of the configuration at a chiral carbon atom was impossible.
With this starting point, all related chiral compounds could be chemically transformed into (−)- or (+)-glyceraldehyde. By employing only chemical transformations with retention of stereochemical configuration, such an unknown chiral compound could be assigned either a D- or an L-configuration.
The use of vanadium pentoxide for the catalytic oxidation with air of various substances became his most profitable invention. Similar catalysts are still used for the oxidation of naphthalene
, anthraquinone
and for the production of sulfuric acid
from sulfur dioxide
.
Germany
Germany , officially the Federal Republic of Germany , is a federal parliamentary republic in Europe. The country consists of 16 states while the capital and largest city is Berlin. Germany covers an area of 357,021 km2 and has a largely temperate seasonal climate...
chemist
Chemist
A chemist is a scientist trained in the study of chemistry. Chemists study the composition of matter and its properties such as density and acidity. Chemists carefully describe the properties they study in terms of quantities, with detail on the level of molecules and their component atoms...
.
Several chemical reactions are named after him, including the Wohl degradation
Wohl degradation
The Wohl degradation in carbohydrate chemistry is a chain contraction method for aldoses. The classic example is the conversion of glucose to arabinose as shown below. The reaction is named after the chemist Alfred Wohl....
, Wohl-Aue reaction
Wohl-Aue reaction
The Wohl-Aue reaction is an organic reaction between an aromatic nitro compound and an aniline to form a phenazine in presence of an alkali base. An example is the reaction between nitrobenzene and aniline:The reaction is named after Alfred Wohl and W. Aue....
and the Wohl-Ziegler reaction
Wohl-Ziegler reaction
The Wohl-Ziegler reactionis a chemical reaction that involves the allylic or benzylic bromination of hydrocarbons using an N-bromoimide and a radical initiator.Best yields are achieved with N-bromosuccinimide in carbon tetrachloride solvent...
.
Life
Wohl studied chemistry at the University of Heidelberg from 1882 until 1886. He received his Ph.D 1886 for work on Hexamethylenetetramine with August Wilhelm von HofmannAugust Wilhelm von Hofmann
August Wilhelm von Hofmann was a German chemist.-Biography:Hofmann was born at Gießen, Grand Duchy of Hesse. Not intending originally to devote himself to physical science, he first took up the study of law and philology at Göttingen. But he then turned to chemistry, and studied under Justus von...
. He became an assistant of Hermann Emil Fischer
Hermann Emil Fischer
Hermann Emil Fischer, Emil Fischer was a German chemist and 1902 recipient of the Nobel Prize in Chemistry. He discovered the Fischer esterification. He developed the Fischer projection, a symbolic way of drawing asymmetric carbon atoms.-Early years:Fischer was born in Euskirchen, near Cologne,...
at the University of Berlin from 1886 until 1891, where he also received his habilitation
Habilitation
Habilitation is the highest academic qualification a scholar can achieve by his or her own pursuit in several European and Asian countries. Earned after obtaining a research doctorate, such as a PhD, habilitation requires the candidate to write a professorial thesis based on independent...
. He became professor at the University of Berlin in 1901, but he left for the Technical University of Danzig in 1904. He retired because of antisemitic pressure in 1933, but worked in his lab until 1937. He emigrated to Sweden in 1938, where he died in 1939.
Work
His work in organic chemistry started with his PhD thesis on hexamineHexamine
Hexamethylenetetramine is a heterocyclic organic compound with the formula 6N4. This white crystalline compound is highly soluble in water and polar organic solvents. It has a cage-like structure similar to adamantane. It is useful in the synthesis of other chemical compounds, e.g. plastics,...
in 1886. Under the influence of Fischer, Wohl focused on sugar chemistry. Wohl arbitrarily defined the structure of (+)-glyceraldehyde to have the D-configuration, forming the basis for the D-L system. This was done before chemists had the ability to prove (+)-glyceraldehyde was, in fact, D-glyceraldehyde. Before the invention of x-ray crystallography
X-ray crystallography
X-ray crystallography is a method of determining the arrangement of atoms within a crystal, in which a beam of X-rays strikes a crystal and causes the beam of light to spread into many specific directions. From the angles and intensities of these diffracted beams, a crystallographer can produce a...
the exact determination of the configuration at a chiral carbon atom was impossible.
| D-glyceraldehyde |
L-glyceraldehyde |
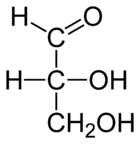 |
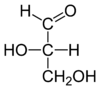 |
With this starting point, all related chiral compounds could be chemically transformed into (−)- or (+)-glyceraldehyde. By employing only chemical transformations with retention of stereochemical configuration, such an unknown chiral compound could be assigned either a D- or an L-configuration.
The use of vanadium pentoxide for the catalytic oxidation with air of various substances became his most profitable invention. Similar catalysts are still used for the oxidation of naphthalene
Naphthalene
Naphthalene is an organic compound with formula . It is a white crystalline solid with a characteristic odor that is detectable at concentrations as low as 0.08 ppm by mass. As an aromatic hydrocarbon, naphthalene's structure consists of a fused pair of benzene rings...
, anthraquinone
Anthraquinone
Anthraquinone, also called anthracenedione or dioxoanthracene is an aromatic organic compound with formula . Several isomers are possible, each of which can be viewed as a quinone derivative...
and for the production of sulfuric acid
Sulfuric acid
Sulfuric acid is a strong mineral acid with the molecular formula . Its historical name is oil of vitriol. Pure sulfuric acid is a highly corrosive, colorless, viscous liquid. The salts of sulfuric acid are called sulfates...
from sulfur dioxide
Sulfur dioxide
Sulfur dioxide is the chemical compound with the formula . It is released by volcanoes and in various industrial processes. Since coal and petroleum often contain sulfur compounds, their combustion generates sulfur dioxide unless the sulfur compounds are removed before burning the fuel...
.

