Zirconium diboride
Encyclopedia
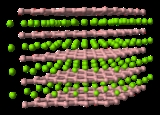
Zirconium diboride is a highly covalent refractory ceramic material with a hexagonal crystal structure. ZrB2 is an Ultra High Temperature Ceramic (UHTC) with a melting point of 3246 °C. This along with its relatively low density of ~6.09 g/cm3 (measured density may be higher due to hafnium
impurities) and good high temperature strength makes it a candidate for high temperature aerospace applications such as hypersonic flight or rocket propulsion systems.
ZrB2 parts are usually hot pressed
(pressure applied to the heated powder) and then machined to shape. Sintering of ZrB2 is hindered by the material's covalent nature and presence of surface oxides which increase grain coarsening before densification during sintering
. Pressureless sintering of ZrB2 is possible with sintering additives such as boron carbide
and carbon
which react with the surface oxides to increase the driving force for sintering but mechanical properties are degraded compared to hot pressed ZrB2.
Additions of ~30 vol% SiC to ZrB2 is often added to ZrB2 to improve oxidation resistance through SiC creating a protective oxide layer - similar to aluminum's protective alumina layer.
The layered bonding between each layer is also very strong but means that the ceramic is highly anisotropic, having different thermal expansions in the 'z' <001> direction. Although the material has excellent high temperature properties, the ceramic has to be produced extremely carefully as any excess of either zirconium or boron will not be accommodated in the ZrB2 lattice (ie. the material does not deviate from stoichiometry). Instead it will form extra lower melting point phases which may initiate failure under extreme conditions.
Hafnium
Hafnium is a chemical element with the symbol Hf and atomic number 72. A lustrous, silvery gray, tetravalent transition metal, hafnium chemically resembles zirconium and is found in zirconium minerals. Its existence was predicted by Dmitri Mendeleev in 1869. Hafnium was the penultimate stable...
impurities) and good high temperature strength makes it a candidate for high temperature aerospace applications such as hypersonic flight or rocket propulsion systems.
ZrB2 parts are usually hot pressed
Hot pressing
Hot pressing is a high-pressure, low-strain-rate powder metallurgy process for forming of a powder or powder compact at a temperature high enough to induce sintering and creep processes. This is achieved by the simultaneous application of heat and pressure....
(pressure applied to the heated powder) and then machined to shape. Sintering of ZrB2 is hindered by the material's covalent nature and presence of surface oxides which increase grain coarsening before densification during sintering
Sintering
Sintering is a method used to create objects from powders. It is based on atomic diffusion. Diffusion occurs in any material above absolute zero, but it occurs much faster at higher temperatures. In most sintering processes, the powdered material is held in a mold and then heated to a temperature...
. Pressureless sintering of ZrB2 is possible with sintering additives such as boron carbide
Boron carbide
Boron carbide is an extremely hard boron–carbon ceramic material used in tank armor, bulletproof vests, and numerous industrial applications...
and carbon
Carbon
Carbon is the chemical element with symbol C and atomic number 6. As a member of group 14 on the periodic table, it is nonmetallic and tetravalent—making four electrons available to form covalent chemical bonds...
which react with the surface oxides to increase the driving force for sintering but mechanical properties are degraded compared to hot pressed ZrB2.
Additions of ~30 vol% SiC to ZrB2 is often added to ZrB2 to improve oxidation resistance through SiC creating a protective oxide layer - similar to aluminum's protective alumina layer.
Defects and secondary phases in zirconium diboride
Zirconium diboride gains its high temperature mechanical stability from the high atomic defect energies (ie. the atoms do not deviate easily from their lattice sites). This means that the concentration of defects will remain low, even at high temperatures, preventing failure of the material.The layered bonding between each layer is also very strong but means that the ceramic is highly anisotropic, having different thermal expansions in the 'z' <001> direction. Although the material has excellent high temperature properties, the ceramic has to be produced extremely carefully as any excess of either zirconium or boron will not be accommodated in the ZrB2 lattice (ie. the material does not deviate from stoichiometry). Instead it will form extra lower melting point phases which may initiate failure under extreme conditions.

