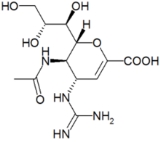
Zanamivir
Encyclopedia
Zanamivir INN
(icon) is a neuraminidase inhibitor
used in the treatment and prophylaxis of influenza
caused by influenza A virus and influenza B virus. Zanamivir was the first neuraminidase inhibitor commercially developed. It is currently marketed by GlaxoSmithKline
under the trade name
Relenza as a powder for oral inhalation.
According to the Centers for Disease Control and Prevention
(CDC), no flu, seasonal or pandemic, has shown any signs of resistance to zanamivir.
, in collaboration with the CSIRO and scientists at Glaxo, UK. Zanamivir was the first of the neuraminidase inhibitor
s. The discovery was initially funded by the Australian biotechnology company Biota
and was part of Biota's ongoing program to develop antiviral agents through rational drug design. Its strategy relied on the availability of the structure of influenza neuraminidase
, by X-ray crystallography
. It was also known, as far back as 1974, that 2-deoxy-2,3-didehydro-N-acetylneuraminic acid (DANA), a sialic acid
analogue, was an inhibitor of neuraminidase. Sialic acid (N-acetyl neuraminic acid, NANA), the substrate of neuraminidase, is itself a mild inhibitor of the enzyme, but the dehydrated derivative DANA, a transition-state analogue, is a better inhibitor.
Computational chemistry
techniques were used to probe the active site of the enzyme, in an attempt to design derivatives of DANA that would bind tightly to the aminoacid residues of the catalytic site, and so would be potent and specific inhibitors of the enzyme. The GRID software by Molecular Discovery
was used to determine energetically favourable interactions between various functional groups and residues in the catalytic site canyon. This showed there was a negatively charged zone in the neuraminidase active site that aligned with the C4 hydroxyl group of DANA. This hydroxyl was therefore replaced with a positively charged amino group; the 4-amino DANA was 100 times better an inhibitor than DANA, owing to the formation of a salt bridge with a conserved glutamic acid (119) in the active site. It was also noticed that Glu 119 was at the bottom of a conserved pocket in the active site just big enough to accommodate a more basic functional positively charged group, such as a guanidino group, which was also larger than the amino group. Zanamivir, a transition-state analogue inhibitor of neuraminidase, was the result.
As Biota was a small company, it did not have the resources to bring zanamivir to market by itself. In 1990, zanamivir patent rights were licensed to Glaxo, now GlaxoSmithKline
(GSK). In 1999, the product was approved for marketing in the US and subsequently has been registered by GSK in a total of 70 countries (GlaxoSmithKline News release, 2006). Zanamivir is delivered via Glaxo's proprietary Diskhaler inhalation device. The license agreement entitled Biota to receive a 7% royalty on Glaxo's sales of zanamivir.
In January 2011, GlaxoSmithKline started a pivotal study testing i.v. zanamivir against p.o. oseltamivir (the Tamiflu pill) as a treatment for patients hospitalised with influenza. GSK's press release
As a proven anti-influenza drug target, neuraminidase continues to be attractive for the development of new inhibitors. The crystal structure of H5N1 avian influenza neuraminidase (PDB code: 2HTY) provides the three-dimensional structural information and opportunity for finding new inhibitors in this regard, because the existing inhibitors, such as oseltamivir and zanamivir, were developed based on different structures of neuraminidase, such as subtypes N9, N2, and type B genus of influenza virus.
Influenza, commonly known as the flu, is caused by a virus which targets the body's respiratory cells and damages the lining of the respiratory tract, leading to swelling and inflammation of the tract. Influenza spreads rapidly by replicating itself inside the host cell, producing hundreds of copies of the virus in a short period. In approximately an hour the virus can destroy the host cell and propel its replications out into the body to find new host cells. For some people, the flu and its complications can be very serious, even fatal.
Zanamivir works by binding to the active site
of the neuraminidase protein, rendering the influenza virus unable to escape its host cell and infect others. It is also an inhibitor of influenza virus replication in vitro and in vivo. In clinical trials it was found that zanamivir was able to reduce the time to symptom resolution by 1.5 days if therapy was started within 48 hours of the onset of symptoms.
of zanamivir is 2%. After inhalation, zanamivir is concentrated in the lungs and oropharynx
, where up to 15% of the dose is absorbed and excreted in urine.
Dosing is limited to the inhaled route. This restricts its usage, as treating asthmatics could induce bronchospasm
. The U.S. Food and Drug Administration (FDA) has issued a Public Health Advisory warning that it has received some reports of respiratory problems following inhalation of zanamivir by patients with underlying asthma
or chronic obstructive pulmonary disease
. The zanamivir package insert contains precautionary information regarding risk of bronchospasm in patients with respiratory disease.
Zanamivir has not been known to cause toxic effects, does not spread around through the body's systemic circulation and shows no signs of viral resistance from any flu.
GlaxoSmithKline (GSK) and FDA notified healthcare professionals of a report of the death of a patient with influenza who received Relenza (zanamivir) Inhalation Powder which was solubilized and administered by mechanical ventilation.
to the market, it had only a few months lead over the second entrant, oseltamivir
(Tamiflu), with an oral tablet formulation.
According to the CDC, Tamiflu, zanamivir’s main competitor, is not as effective at treating the Influenza viruses as zanamivir, especially in H1N1 Seasonal Flu. In fact, tests showed that 99.6% of the tested strains of seasonal H1N1 flu and 0.5% of 2009 pandemic flu were resistant to Tamiflu while there have been absolutely zero flu samples seasonal or pandemic that show any resistance to zanamivir.
When first marketed in the US in 1999/2000, zanamivir captured only 25% of the influenza antiviral market, despite a huge promotional campaign. By the end of that season, Tamiflu was outselling zanamivir 3:1. During that season, zanamivir experienced worldwide safety warnings involving the risk of bronchospasm and death. Glaxo then reduced the marketing of zanamivir, and Tamiflu's dominance increased. More than US$
20m worth of zanamivir sold by Glaxo in the first US season was returned to the company in the next two seasons because zanamivir's sales to patients were far less than expected.
Biota commenced legal proceedings in 2004 alleging that Glaxo's reduced marketing of zanamivir was a breach of contract. Biota claimed approximately A$
700m from Glaxo. After Biota spent four years trying to progress its case, and incurring A$
50m in legal costs, the company abandoned the claim in July 2008, recovering only A$
20m including legal costs following settlement at mediation. Biota had refused an earlier tactical offer from Glaxo of A$
75m plus legal costs.
In August 2006, Germany announced that it would buy 1.7 million doses of zanamivir, as part of its preparation strategy against bird flu. "Germany's purchase shows that countries are starting to take a balanced view of influenza preparedness," says Simon Tucker, head of research at Melbourne-based Biota, where zanamivir was originally developed.
In April 2009 many cases of swine flu
(H1N1 type virus) were reported in US and Mexico. Zanamivir is one of only two drugs that are prescribed to treat it. A study published in June 2009 emphasized the urgent need for augmentation of oseltamivir
(Tamiflu) stockpiles, with additional antiviral drugs including zanamivir, based on an evaluation of the performance of these drugs in the scenario that the 2009 H1N1 swine flu neuraminidase (NA) were to acquire the Tamiflu-resistance (His274Tyr) mutation which is currently widespread in 99.6% of all tested seasonal H1N1 strains.
In January 2011, GSK announced it was commencing Phase III trials for Intravenous zanamivir in a study which will span 20 countries in the Northern and Southern hemispheres.
It is not recommended for people with respiratory problems and ailments.
International Nonproprietary Name
An International Nonproprietary Name is the official nonproprietary or generic name given to a pharmaceutical substance, as designated by the World Health Organization...
(icon) is a neuraminidase inhibitor
Neuraminidase inhibitor
Neuraminidase inhibitors are a class of antiviral drugs targeted at the influenza virus, which work by blocking the function of the viral neuraminidase protein, thus preventing the virus from reproducing by budding from the host cell....
used in the treatment and prophylaxis of influenza
Influenza
Influenza, commonly referred to as the flu, is an infectious disease caused by RNA viruses of the family Orthomyxoviridae , that affects birds and mammals...
caused by influenza A virus and influenza B virus. Zanamivir was the first neuraminidase inhibitor commercially developed. It is currently marketed by GlaxoSmithKline
GlaxoSmithKline
GlaxoSmithKline plc is a global pharmaceutical, biologics, vaccines and consumer healthcare company headquartered in London, United Kingdom...
under the trade name
Trade name
A trade name, also known as a trading name or a business name, is the name which a business trades under for commercial purposes, although its registered, legal name, used for contracts and other formal situations, may be another....
Relenza as a powder for oral inhalation.
According to the Centers for Disease Control and Prevention
Centers for Disease Control and Prevention
The Centers for Disease Control and Prevention are a United States federal agency under the Department of Health and Human Services headquartered in Druid Hills, unincorporated DeKalb County, Georgia, in Greater Atlanta...
(CDC), no flu, seasonal or pandemic, has shown any signs of resistance to zanamivir.
History
Zanamivir was discovered in 1989 by scientists led by Mark von Itzstein at the Victorian College of Pharmacy, Monash UniversityMonash University
Monash University is a public university based in Melbourne, Victoria. It was founded in 1958 and is the second oldest university in the state. Monash is a member of Australia's Group of Eight and the ASAIHL....
, in collaboration with the CSIRO and scientists at Glaxo, UK. Zanamivir was the first of the neuraminidase inhibitor
Neuraminidase inhibitor
Neuraminidase inhibitors are a class of antiviral drugs targeted at the influenza virus, which work by blocking the function of the viral neuraminidase protein, thus preventing the virus from reproducing by budding from the host cell....
s. The discovery was initially funded by the Australian biotechnology company Biota
Biota Holdings
Biota is an Australian antiviral drug development company listed on the Australian Stock Exchange .In 1989 Biota discovered the drug Zanamivir, which acts as a neuraminidase inhibitor for the treatment and prevention of influenza. This drug is licensed to GlaxoSmithKline and marketed as Relenza....
and was part of Biota's ongoing program to develop antiviral agents through rational drug design. Its strategy relied on the availability of the structure of influenza neuraminidase
Neuraminidase
Neuraminidase enzymes are glycoside hydrolase enzymes that cleave the glycosidic linkages of neuraminic acids. Neuraminidase enzymes are a large family, found in a range of organisms. The most commonly known neuraminidase is the viral neuraminidase, a drug target for the prevention of the spread...
, by X-ray crystallography
X-ray crystallography
X-ray crystallography is a method of determining the arrangement of atoms within a crystal, in which a beam of X-rays strikes a crystal and causes the beam of light to spread into many specific directions. From the angles and intensities of these diffracted beams, a crystallographer can produce a...
. It was also known, as far back as 1974, that 2-deoxy-2,3-didehydro-N-acetylneuraminic acid (DANA), a sialic acid
Sialic acid
Sialic acid is a generic term for the N- or O-substituted derivatives of neuraminic acid, a monosaccharide with a nine-carbon backbone. It is also the name for the most common member of this group, N-acetylneuraminic acid...
analogue, was an inhibitor of neuraminidase. Sialic acid (N-acetyl neuraminic acid, NANA), the substrate of neuraminidase, is itself a mild inhibitor of the enzyme, but the dehydrated derivative DANA, a transition-state analogue, is a better inhibitor.
Computational chemistry
Computational chemistry
Computational chemistry is a branch of chemistry that uses principles of computer science to assist in solving chemical problems. It uses the results of theoretical chemistry, incorporated into efficient computer programs, to calculate the structures and properties of molecules and solids...
techniques were used to probe the active site of the enzyme, in an attempt to design derivatives of DANA that would bind tightly to the aminoacid residues of the catalytic site, and so would be potent and specific inhibitors of the enzyme. The GRID software by Molecular Discovery
Molecular Discovery
Molecular Discovery Ltd is a software company working in the area of drug discovery.Founded in 1984 by Peter Goodford, its aim was to provide the GRID software to scientists working in the field of Drug Design, and enabled one of the first examples of rational drug design with the discovery of...
was used to determine energetically favourable interactions between various functional groups and residues in the catalytic site canyon. This showed there was a negatively charged zone in the neuraminidase active site that aligned with the C4 hydroxyl group of DANA. This hydroxyl was therefore replaced with a positively charged amino group; the 4-amino DANA was 100 times better an inhibitor than DANA, owing to the formation of a salt bridge with a conserved glutamic acid (119) in the active site. It was also noticed that Glu 119 was at the bottom of a conserved pocket in the active site just big enough to accommodate a more basic functional positively charged group, such as a guanidino group, which was also larger than the amino group. Zanamivir, a transition-state analogue inhibitor of neuraminidase, was the result.
As Biota was a small company, it did not have the resources to bring zanamivir to market by itself. In 1990, zanamivir patent rights were licensed to Glaxo, now GlaxoSmithKline
GlaxoSmithKline
GlaxoSmithKline plc is a global pharmaceutical, biologics, vaccines and consumer healthcare company headquartered in London, United Kingdom...
(GSK). In 1999, the product was approved for marketing in the US and subsequently has been registered by GSK in a total of 70 countries (GlaxoSmithKline News release, 2006). Zanamivir is delivered via Glaxo's proprietary Diskhaler inhalation device. The license agreement entitled Biota to receive a 7% royalty on Glaxo's sales of zanamivir.
Developments
Recently, the reported oseltamivir-resistance H5N1 virus neuraminidase still retaining susceptibility to zanamivir indicates that the structure of zanamivir has some advantages over oseltamivir in binding to the active pocket of H5N1 neuraminidase.In January 2011, GlaxoSmithKline started a pivotal study testing i.v. zanamivir against p.o. oseltamivir (the Tamiflu pill) as a treatment for patients hospitalised with influenza. GSK's press release
As a proven anti-influenza drug target, neuraminidase continues to be attractive for the development of new inhibitors. The crystal structure of H5N1 avian influenza neuraminidase (PDB code: 2HTY) provides the three-dimensional structural information and opportunity for finding new inhibitors in this regard, because the existing inhibitors, such as oseltamivir and zanamivir, were developed based on different structures of neuraminidase, such as subtypes N9, N2, and type B genus of influenza virus.
Pharmacology
Zanamivir is specifically for the treatment of infections caused by Influenzavirus A and Influenzavirus B.Influenza, commonly known as the flu, is caused by a virus which targets the body's respiratory cells and damages the lining of the respiratory tract, leading to swelling and inflammation of the tract. Influenza spreads rapidly by replicating itself inside the host cell, producing hundreds of copies of the virus in a short period. In approximately an hour the virus can destroy the host cell and propel its replications out into the body to find new host cells. For some people, the flu and its complications can be very serious, even fatal.
Zanamivir works by binding to the active site
Active site
In biology the active site is part of an enzyme where substrates bind and undergo a chemical reaction. The majority of enzymes are proteins but RNA enzymes called ribozymes also exist. The active site of an enzyme is usually found in a cleft or pocket that is lined by amino acid residues that...
of the neuraminidase protein, rendering the influenza virus unable to escape its host cell and infect others. It is also an inhibitor of influenza virus replication in vitro and in vivo. In clinical trials it was found that zanamivir was able to reduce the time to symptom resolution by 1.5 days if therapy was started within 48 hours of the onset of symptoms.
Dosing and side effects
The bioavailabilityBioavailability
In pharmacology, bioavailability is a subcategory of absorption and is used to describe the fraction of an administered dose of unchanged drug that reaches the systemic circulation, one of the principal pharmacokinetic properties of drugs. By definition, when a medication is administered...
of zanamivir is 2%. After inhalation, zanamivir is concentrated in the lungs and oropharynx
Oropharynx
The Oropharynx reaches from the Uvula to the level of the hyoid bone.It opens anteriorly, through the isthmus faucium, into the mouth, while in its lateral wall, between the two palatine arches, is the palatine tonsil....
, where up to 15% of the dose is absorbed and excreted in urine.
Dosing is limited to the inhaled route. This restricts its usage, as treating asthmatics could induce bronchospasm
Bronchospasm
Bronchospasm or a bronchial spasm is a sudden constriction of the muscles in the walls of the bronchioles. It is caused by the release of substances from mast cells or basophils under the influence of anaphylatoxins...
. The U.S. Food and Drug Administration (FDA) has issued a Public Health Advisory warning that it has received some reports of respiratory problems following inhalation of zanamivir by patients with underlying asthma
Asthma
Asthma is the common chronic inflammatory disease of the airways characterized by variable and recurring symptoms, reversible airflow obstruction, and bronchospasm. Symptoms include wheezing, coughing, chest tightness, and shortness of breath...
or chronic obstructive pulmonary disease
Chronic obstructive pulmonary disease
Chronic obstructive pulmonary disease , also known as chronic obstructive lung disease , chronic obstructive airway disease , chronic airflow limitation and chronic obstructive respiratory disease , is the co-occurrence of chronic bronchitis and emphysema, a pair of commonly co-existing diseases...
. The zanamivir package insert contains precautionary information regarding risk of bronchospasm in patients with respiratory disease.
Zanamivir has not been known to cause toxic effects, does not spread around through the body's systemic circulation and shows no signs of viral resistance from any flu.
GlaxoSmithKline (GSK) and FDA notified healthcare professionals of a report of the death of a patient with influenza who received Relenza (zanamivir) Inhalation Powder which was solubilized and administered by mechanical ventilation.
Commercial issues
Although zanamivir was the first neuraminidase inhibitorNeuraminidase inhibitor
Neuraminidase inhibitors are a class of antiviral drugs targeted at the influenza virus, which work by blocking the function of the viral neuraminidase protein, thus preventing the virus from reproducing by budding from the host cell....
to the market, it had only a few months lead over the second entrant, oseltamivir
Oseltamivir
Oseltamivir INN , an antiviral drug, slows the spread of influenza virus between cells in the body by stopping the virus from chemically cutting ties with its host cell; median time to symptom alleviation is reduced by 0.5–1 day. The drug is sold under the trade name Tamiflu, and is taken orally...
(Tamiflu), with an oral tablet formulation.
According to the CDC, Tamiflu, zanamivir’s main competitor, is not as effective at treating the Influenza viruses as zanamivir, especially in H1N1 Seasonal Flu. In fact, tests showed that 99.6% of the tested strains of seasonal H1N1 flu and 0.5% of 2009 pandemic flu were resistant to Tamiflu while there have been absolutely zero flu samples seasonal or pandemic that show any resistance to zanamivir.
When first marketed in the US in 1999/2000, zanamivir captured only 25% of the influenza antiviral market, despite a huge promotional campaign. By the end of that season, Tamiflu was outselling zanamivir 3:1. During that season, zanamivir experienced worldwide safety warnings involving the risk of bronchospasm and death. Glaxo then reduced the marketing of zanamivir, and Tamiflu's dominance increased. More than US$
United States dollar
The United States dollar , also referred to as the American dollar, is the official currency of the United States of America. It is divided into 100 smaller units called cents or pennies....
20m worth of zanamivir sold by Glaxo in the first US season was returned to the company in the next two seasons because zanamivir's sales to patients were far less than expected.
Biota commenced legal proceedings in 2004 alleging that Glaxo's reduced marketing of zanamivir was a breach of contract. Biota claimed approximately A$
Australian dollar
The Australian dollar is the currency of the Commonwealth of Australia, including Christmas Island, Cocos Islands, and Norfolk Island, as well as the independent Pacific Island states of Kiribati, Nauru and Tuvalu...
700m from Glaxo. After Biota spent four years trying to progress its case, and incurring A$
Australian dollar
The Australian dollar is the currency of the Commonwealth of Australia, including Christmas Island, Cocos Islands, and Norfolk Island, as well as the independent Pacific Island states of Kiribati, Nauru and Tuvalu...
50m in legal costs, the company abandoned the claim in July 2008, recovering only A$
Australian dollar
The Australian dollar is the currency of the Commonwealth of Australia, including Christmas Island, Cocos Islands, and Norfolk Island, as well as the independent Pacific Island states of Kiribati, Nauru and Tuvalu...
20m including legal costs following settlement at mediation. Biota had refused an earlier tactical offer from Glaxo of A$
Australian dollar
The Australian dollar is the currency of the Commonwealth of Australia, including Christmas Island, Cocos Islands, and Norfolk Island, as well as the independent Pacific Island states of Kiribati, Nauru and Tuvalu...
75m plus legal costs.
In August 2006, Germany announced that it would buy 1.7 million doses of zanamivir, as part of its preparation strategy against bird flu. "Germany's purchase shows that countries are starting to take a balanced view of influenza preparedness," says Simon Tucker, head of research at Melbourne-based Biota, where zanamivir was originally developed.
In April 2009 many cases of swine flu
2009 flu pandemic
The 2009 flu pandemic was an influenza pandemic, and the second of the two pandemics involving H1N1 influenza virus , albeit in a new version...
(H1N1 type virus) were reported in US and Mexico. Zanamivir is one of only two drugs that are prescribed to treat it. A study published in June 2009 emphasized the urgent need for augmentation of oseltamivir
Oseltamivir
Oseltamivir INN , an antiviral drug, slows the spread of influenza virus between cells in the body by stopping the virus from chemically cutting ties with its host cell; median time to symptom alleviation is reduced by 0.5–1 day. The drug is sold under the trade name Tamiflu, and is taken orally...
(Tamiflu) stockpiles, with additional antiviral drugs including zanamivir, based on an evaluation of the performance of these drugs in the scenario that the 2009 H1N1 swine flu neuraminidase (NA) were to acquire the Tamiflu-resistance (His274Tyr) mutation which is currently widespread in 99.6% of all tested seasonal H1N1 strains.
In January 2011, GSK announced it was commencing Phase III trials for Intravenous zanamivir in a study which will span 20 countries in the Northern and Southern hemispheres.
Legal status
The drug is approved for use for the prevention and treatment of influenza in those over the age of 7 in the United States, Canada, European Union and many other countries.It is not recommended for people with respiratory problems and ailments.
External links
- The development of Relenza for treatment of influenza
- Drug Information: Zanamivir Inhalation MedlinePlus drug
- Relenza: Consumer Questions and Answers US Food and Drug Administration (FDA)
- http://www.biota.com.au/?page=1021002&subpage=1021104Relenza Biota HoldingsBiota HoldingsBiota is an Australian antiviral drug development company listed on the Australian Stock Exchange .In 1989 Biota discovered the drug Zanamivir, which acts as a neuraminidase inhibitor for the treatment and prevention of influenza. This drug is licensed to GlaxoSmithKline and marketed as Relenza....
] - Relenza web site GlaxoSmithKlineGlaxoSmithKlineGlaxoSmithKline plc is a global pharmaceutical, biologics, vaccines and consumer healthcare company headquartered in London, United Kingdom...

