Virulence-related outer membrane protein family
Encyclopedia
Virulence-related outer membrane proteins are expressed in Gram-negative bacteria and are essential to bacterial survival within macrophages and for eukaryotic cell invasion.
This family consists of several bacterial and phage Ail/Lom-like proteins.
The Yersinia enterocolitica Ail protein is a known virulence factor.
Proteins in this family are predicted to consist of eight transmembrane
beta-sheets and four cell surface-exposed loops. It is thought that Ail
directly promotes invasion and loop 2 contains an active site, perhaps a
receptor-binding domain. The phage protein Lom is expressed during
lysogeny, and encode host-cell envelope proteins. Lom is found in the
bacterial outer membrane, and is homologous to virulence proteins of two
other enterobacterial genera. It has been suggested that lysogeny may
generally have a role in bacterial survival in animal hosts, and perhaps in
pathogenesis.
Members of this group include:
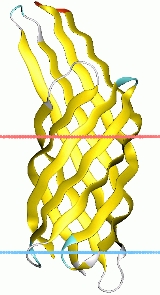
The crystal structure of OmpX from E. coli reveals that OmpX consists of an eight-stranded antiparallel all-next-neighbour beta barrel. The structure shows two girdles of aromatic amino acid residues and a ribbon of nonpolar residues that attach to the membrane interior. The core of the barrel consists of an extended hydrogen-bonding network of highly conserved residues. OmpX thus resembles an inverse micelle. The OmpX structure shows that the membrane-spanning part of the protein is much better conserved than the extracellular loops. Moreover, these loops form a protruding beta sheet, the edge of which presumably binds to external proteins. It is suggested that this type of binding promotes cell adhesion and invasion and helps defend against the complement system. Although OmpX has the same beta-sheet topology as the structurally related outer membrane protein A (OmpA) , their barrels differ with respect to the shear numbers and internal hydrogen-bonding networks.
This family consists of several bacterial and phage Ail/Lom-like proteins.
The Yersinia enterocolitica Ail protein is a known virulence factor.
Proteins in this family are predicted to consist of eight transmembrane
beta-sheets and four cell surface-exposed loops. It is thought that Ail
directly promotes invasion and loop 2 contains an active site, perhaps a
receptor-binding domain. The phage protein Lom is expressed during
lysogeny, and encode host-cell envelope proteins. Lom is found in the
bacterial outer membrane, and is homologous to virulence proteins of two
other enterobacterial genera. It has been suggested that lysogeny may
generally have a role in bacterial survival in animal hosts, and perhaps in
pathogenesis.
Members of this group include:
- PagC, required by Salmonella typhimurium for survival in macrophages and for virulence in mice
- Rck outer membrane protein of the S. typhimurium virulence plasmid
- Ail, a product of the Yersinia enterocoliticaYersinia enterocoliticaYersinia enterocolitica is a species of gram-negative coccobacillus-shaped bacterium, belonging to the family Enterobacteriaceae. Yersinia enterocolitica infection causes the disease yersiniosis, which is a zoonotic disease occurring in humans as well as a wide array of animals such as cattle,...
chromosome capable of mediating bacterial adherence to and invasion of epithelial cell lines - OmpX from Escherichia coliEscherichia coliEscherichia coli is a Gram-negative, rod-shaped bacterium that is commonly found in the lower intestine of warm-blooded organisms . Most E. coli strains are harmless, but some serotypes can cause serious food poisoning in humans, and are occasionally responsible for product recalls...
that promotes adhesion to and entry into mammalian cells. It also has a role in the resistance against attack by the human complement system - a Bacteriophage lambda outer membrane protein, Lom
The crystal structure of OmpX from E. coli reveals that OmpX consists of an eight-stranded antiparallel all-next-neighbour beta barrel. The structure shows two girdles of aromatic amino acid residues and a ribbon of nonpolar residues that attach to the membrane interior. The core of the barrel consists of an extended hydrogen-bonding network of highly conserved residues. OmpX thus resembles an inverse micelle. The OmpX structure shows that the membrane-spanning part of the protein is much better conserved than the extracellular loops. Moreover, these loops form a protruding beta sheet, the edge of which presumably binds to external proteins. It is suggested that this type of binding promotes cell adhesion and invasion and helps defend against the complement system. Although OmpX has the same beta-sheet topology as the structurally related outer membrane protein A (OmpA) , their barrels differ with respect to the shear numbers and internal hydrogen-bonding networks.
Further reading
- Identification of regions of Ail required for the invasion and serum resistance phenotypes. Miller VL, Beer KB, Heusipp G, Young BM, Wachtel MR; Mol Microbiol 2001;41:1053-1062.
- A bacterial virulence determinant encoded by lysogenic coliphage lambda. Barondess JJ, Beckwith J; Nature 1990;346:871-874.

