
Vegard's law
Encyclopedia
In metallurgy
, Vegard's law is an approximate empirical rule which holds that a linear relation exists, at constant temperature, between the crystal lattice parameter of an alloy
and the concentrations of the constituent elements.
For example, consider the semiconductor compound InPxAs1-x. A relation exists between the constituent elements and their associated lattice parameters, , such that:
, such that:
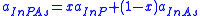
One can also extend this relation to determine semiconductor band gap energies. Using InPxAs1-x as before one can find an expression that relates the band gap energies,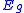 , to the ratio of the constituents and a bowing parameter
, to the ratio of the constituents and a bowing parameter 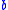 :
:
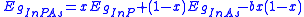
When variations in lattice parameter are very small across the entire composition range, Vegard's law becomes equivalent to Amagat's law
.
Metallurgy
Metallurgy is a domain of materials science that studies the physical and chemical behavior of metallic elements, their intermetallic compounds, and their mixtures, which are called alloys. It is also the technology of metals: the way in which science is applied to their practical use...
, Vegard's law is an approximate empirical rule which holds that a linear relation exists, at constant temperature, between the crystal lattice parameter of an alloy
Alloy
An alloy is a mixture or metallic solid solution composed of two or more elements. Complete solid solution alloys give single solid phase microstructure, while partial solutions give two or more phases that may or may not be homogeneous in distribution, depending on thermal history...
and the concentrations of the constituent elements.
For example, consider the semiconductor compound InPxAs1-x. A relation exists between the constituent elements and their associated lattice parameters,
 , such that:
, such that: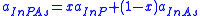
One can also extend this relation to determine semiconductor band gap energies. Using InPxAs1-x as before one can find an expression that relates the band gap energies,
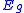 , to the ratio of the constituents and a bowing parameter
, to the ratio of the constituents and a bowing parameter 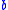 :
: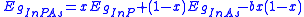
When variations in lattice parameter are very small across the entire composition range, Vegard's law becomes equivalent to Amagat's law
Amagat's law
Amagat's law or the Law of Partial Volumes of 1880 describes the behaviour and properties of mixtures of ideal gases...
.

