
Vapour pressure of water
Encyclopedia
The vapour pressure of water is the pressure at which steam
is saturated
. At higher pressures water would condense. Vapour pressure is a function of temperature. In a gas mixture saturated with water vapour, the vapour pressure is equal to the partial pressure
.
The vapour pressure of water may be approximated by the following relations (in order of increasing accuracy):
.
Steam
Steam is the technical term for water vapor, the gaseous phase of water, which is formed when water boils. In common language it is often used to refer to the visible mist of water droplets formed as this water vapor condenses in the presence of cooler air...
is saturated
Saturation (chemistry)
In chemistry, saturation has six different meanings, all based on reaching a maximum capacity...
. At higher pressures water would condense. Vapour pressure is a function of temperature. In a gas mixture saturated with water vapour, the vapour pressure is equal to the partial pressure
Partial pressure
In a mixture of ideal gases, each gas has a partial pressure which is the pressure which the gas would have if it alone occupied the volume. The total pressure of a gas mixture is the sum of the partial pressures of each individual gas in the mixture....
.
The vapour pressure of water may be approximated by the following relations (in order of increasing accuracy):
- where P is the vapour pressure (mmHg) and T is the temperature in kelvinKelvinThe kelvin is a unit of measurement for temperature. It is one of the seven base units in the International System of Units and is assigned the unit symbol K. The Kelvin scale is an absolute, thermodynamic temperature scale using as its null point absolute zero, the temperature at which all...
.
- Using the Antoine equation

- where the temperature T is in degrees CelsiusCelsiusCelsius is a scale and unit of measurement for temperature. It is named after the Swedish astronomer Anders Celsius , who developed a similar temperature scale two years before his death...
and the vapour pressure P is in mmHg. The constants are given as
| A | B | C | T min. °C |
T max °C |
|
|---|---|---|---|---|---|
| Water | 8.07131 | 1730.63 | 233.426 | 1 | 100 |
| Water | 8.14019 | 1810.94 | 244.485 | 99 | 374 |
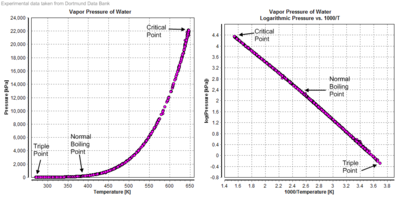
Graphical pressure dependency on temperature

Table of Water Vapour Pressures
The following table list water vapour pressure in units of kPa and mmHg as a function of temperature in degrees CelsiusCelsius
Celsius is a scale and unit of measurement for temperature. It is named after the Swedish astronomer Anders Celsius , who developed a similar temperature scale two years before his death...
.
| Temperature (°C) | Vapour pressure (kPa) | Vapour pressure (mmHg) |
|---|---|---|
| 0 | 0.6 | 4.5 |
| 3 | 0.8 | 6.0 |
| 5 | 0.9 | 6.8 |
| 8 | 1.1 | 8.3 |
| 10 | 1.2 | 9.0 |
| 12 | 1.4 | 10.5 |
| 14 | 1.6 | 12.0 |
| 16 | 1.8 | 13.5 |
| 18 | 2.1 | 15.8 |
| 19 | 2.2 | 16.5 |
| 20 | 2.3 | 17.3 |
| 21 | 2.5 | 18.8 |
| 22 | 2.6 | 19.5 |
| 23 | 2.8 | 21.0 |
| 24 | 3.0 | 22.5 |
| 25 | 3.2 | 24.0 |
| 26 | 3.4 | 25.5 |
| 27 | 3.6 | 27.0 |
| 28 | 3.8 | 28.5 |
| 29 | 4.0 | 30.0 |
| 30 | 4.2 | 31.5 |
| 32 | 4.8 | 36.0 |
| 35 | 5.6 | 42.0 |
| 40 | 7.4 | 55.5 |
| 50 | 12.3 | 92.3 |
| 60 | 19.9 | 149.3 |
| 70 | 31.2 | 234.1 |
| 80 | 47.3 | 354.9 |
| 90 | 70.1 | 525.9 |
| 100 | 101.3 | 760.0 |
Further reading
- Murphy, D. M. and Koop, T. (2005): Review of the vapour pressures of ice and supercooled water for atmospheric applications, Quarterly Journal of the Royal Meteorological Society 131(608): 1539-1565. doi:10.1256/qj.04.94


