
Ununtrium
Encyclopedia
Ununtrium is the temporary name of a synthetic element
with the temporary symbol Uut and atomic number
113.
It is placed as the heaviest member of the group 13 (IIIA) elements although a sufficiently stable isotope
is not known at this time that would allow chemical experiments to confirm its position. It was first detected in 2003 in the decay of ununpentium
and was synthesized directly in 2004. Only fourteen atoms of ununtrium have been observed to date. The longest-lived isotope known is 286Uut with a half-life
of ~20 s, allowing first chemical experiments to study its chemistry.
. These results were published on February 1, 2004, by a team composed of Russian scientists at Dubna
(Joint Institute for Nuclear Research
), and American scientists at the Lawrence Livermore National Laboratory
.

On July 23, 2004, a team of Japanese scientists at RIKEN
detected a single atom of 278Uut using the cold fusion reaction between bismuth-209 and zinc-70. They published their results on September 28, 2004.
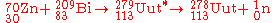
Support for their claim appeared in 2004 when scientists at the Institute of Modern Physics (IMP) identified 266Bh as decaying with identical properties to their single event (see bohrium
).
The RIKEN team produced a further atom on April 2, 2005, although the decay data were different from the first chain, and may be due to the formation of a meta-stable isomer.
The Dubna-Livermore collaboration has strengthened their claim for the discovery of ununtrium by conducting chemical experiments on the decay daughter 268Db. In experiments in June 2004 and December 2005, the dubnium isotope was successfully identified by milking the Db fraction and measuring any SF activities. Both the half-life and decay mode were confirmed for the proposed 268Db which lends support to the assignment of Z=115 and Z=113 to the parent and daughter nuclei.
Theoretical estimates of alpha-decay half-lives of alpha-decay chains from element 113 are in good agreement with the experimental data.
. Research scientists usually refer to the element simply as element 113 (or E113).
The following names have been suggested by the above-mentioned teams claiming discovery:
The synthesis of ununtrium was first attempted in 1998 by the team at GSI using the above cold fusion reaction. In two separate runs, they were unable to detect any atoms and calculated a cross section limit of 900 fb
.
They repeated the experiment in 2003 and lowered the limit further to 400 fb.
In late 2003, the emerging team at RIKEN
using their efficient apparatus GARIS attempted the reaction and reached a limit of 140 fb. In December 2003 – August 2004, they resorted to 'brute force' and performed an eight-month-long irradiation in which they increased the sensitivity to 51 fb. They were able to detect a single atom of 278Uut.
They repeated the reaction in several runs in 2005 and were able to synthesize a second atom. They calculated a record-low 31 fb for the cross section for the 2 atoms. The reaction was repeated again in 2006 with two long production runs but no further atoms were detected. This lowered the yield further to the current value of just 23 fb.
In June 2006, the Dubna-Livermore team synthesised ununtrium directly in the "warm" fusion reaction between neptunium-237 and calcium-48 nuclei. Two atoms of 282Uut were detected with a cross section of 900 fb.
and ununseptium
.
DNS = Di-nuclear system; σ = cross section
thallium
Synthetic element
In chemistry, a synthetic element is a chemical element that is too unstable to occur naturally on Earth, and therefore has to be created artificially. So far 30 synthetic elements have been discovered—that is, synthesized...
with the temporary symbol Uut and atomic number
Atomic number
In chemistry and physics, the atomic number is the number of protons found in the nucleus of an atom and therefore identical to the charge number of the nucleus. It is conventionally represented by the symbol Z. The atomic number uniquely identifies a chemical element...
113.
It is placed as the heaviest member of the group 13 (IIIA) elements although a sufficiently stable isotope
Isotope
Isotopes are variants of atoms of a particular chemical element, which have differing numbers of neutrons. Atoms of a particular element by definition must contain the same number of protons but may have a distinct number of neutrons which differs from atom to atom, without changing the designation...
is not known at this time that would allow chemical experiments to confirm its position. It was first detected in 2003 in the decay of ununpentium
Ununpentium
Ununpentium is the temporary name of a synthetic superheavy element in the periodic table that has the temporary symbol Uup and has the atomic number 115....
and was synthesized directly in 2004. Only fourteen atoms of ununtrium have been observed to date. The longest-lived isotope known is 286Uut with a half-life
Half-life
Half-life, abbreviated t½, is the period of time it takes for the amount of a substance undergoing decay to decrease by half. The name was originally used to describe a characteristic of unstable atoms , but it may apply to any quantity which follows a set-rate decay.The original term, dating to...
of ~20 s, allowing first chemical experiments to study its chemistry.
Discovery profile
The first report of ununtrium was in August 2003 when it was identified as a decay product of ununpentiumUnunpentium
Ununpentium is the temporary name of a synthetic superheavy element in the periodic table that has the temporary symbol Uup and has the atomic number 115....
. These results were published on February 1, 2004, by a team composed of Russian scientists at Dubna
Dubna
Dubna is a town in Moscow Oblast, Russia. It has a status of naukograd , being home to the Joint Institute for Nuclear Research, an international nuclear physics research centre and one of the largest scientific foundations in the country. It is also home to MKB Raduga, a defence aerospace company...
(Joint Institute for Nuclear Research
Joint Institute for Nuclear Research
The Joint Institute for Nuclear Research, JINR , in Dubna, Moscow Oblast , Russia, is an international research centre for nuclear sciences, with 5500 staff members, 1200 researchers including 1000 Ph.D.s from eighteen member states The Joint Institute for Nuclear Research, JINR , in Dubna, Moscow...
), and American scientists at the Lawrence Livermore National Laboratory
Lawrence Livermore National Laboratory
The Lawrence Livermore National Laboratory , just outside Livermore, California, is a Federally Funded Research and Development Center founded by the University of California in 1952...
.

On July 23, 2004, a team of Japanese scientists at RIKEN
RIKEN
is a large natural sciences research institute in Japan. Founded in 1917, it now has approximately 3000 scientists on seven campuses across Japan, the main one in Wako, just outside Tokyo...
detected a single atom of 278Uut using the cold fusion reaction between bismuth-209 and zinc-70. They published their results on September 28, 2004.
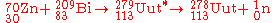
Support for their claim appeared in 2004 when scientists at the Institute of Modern Physics (IMP) identified 266Bh as decaying with identical properties to their single event (see bohrium
Bohrium
Bohrium is a chemical element with the symbol Bh and atomic number 107 and is the heaviest member of group 7 .It is a synthetic element whose most stable known isotope, 270Bh, has a half-life of 61 seconds...
).
The RIKEN team produced a further atom on April 2, 2005, although the decay data were different from the first chain, and may be due to the formation of a meta-stable isomer.
The Dubna-Livermore collaboration has strengthened their claim for the discovery of ununtrium by conducting chemical experiments on the decay daughter 268Db. In experiments in June 2004 and December 2005, the dubnium isotope was successfully identified by milking the Db fraction and measuring any SF activities. Both the half-life and decay mode were confirmed for the proposed 268Db which lends support to the assignment of Z=115 and Z=113 to the parent and daughter nuclei.
Theoretical estimates of alpha-decay half-lives of alpha-decay chains from element 113 are in good agreement with the experimental data.
Naming
The element with atomic number 113 is historically known as eka-thallium. Ununtrium (Uut) is a temporary IUPAC systematic element nameSystematic element name
A systematic element name is the temporary name and symbol assigned to newly synthesized and not yet synthesized chemical elements. In chemistry, a transuranic element receives a permanent name and symbol only after its synthesis has been confirmed. In some cases, this has been a protracted and...
. Research scientists usually refer to the element simply as element 113 (or E113).
Proposed names by claimants
Claims to the discovery of ununtrium have been put forward by Dmitriev of the Dubna team and Morita of the RIKEN team. The IUPAC/IUPAP Joint Working Party will decide to whom the right to suggest a name will be given. In 2011, the IUPAC has evaluated the 2004 RIKEN experiments and 2004 and 2007 Dubna experiments, and concluded that they did not meet the criteria for discovery.The following names have been suggested by the above-mentioned teams claiming discovery:
| Group | Proposed Name | Derivation |
|---|---|---|
| RIKEN | Japonium | Japan: country of group claimants |
| Rikenium | RIKEN: institute of group claimants | |
| Dubna team | Becquerelium | Henri Becquerel Henri Becquerel Antoine Henri Becquerel was a French physicist, Nobel laureate, and the discoverer of radioactivity along with Marie Curie and Pierre Curie, for which all three won the 1903 Nobel Prize in Physics.-Early life:... , French physicist |
Target-projectile combinations leading to Z=113 compound nuclei
The below table contains various combinations of targets and projectiles (both at max no. of neutrons) which could be used to form compound nuclei with an atomic number of 113.| Target | Projectile | CN | Attempt result |
|---|---|---|---|
| 208Pb | 71Ga | 279Uut | |
| 209Bi | 70Zn | 279Uut | |
| 232Th | 51V | 283Uut | |
| 238U | 45Sc | 283Uut | |
| 237Np | 48Ca | 285Uut | |
| 244Pu | 41K | 285Uut | |
| 243Am | 40Ar | 283Uut | |
| 248Cm | 37Cl | 285Uut | |
| 249Bk | 36S | 285Uut | |
| 249Cf | 31P | 280Uut |
Cold fusion
This section deals with the synthesis of nuclei of ununtrium by so-called "cold" fusion reactions. These are processes which create compound nuclei at low excitation energy (~10–20 MeV, hence "cold"), leading to a higher probability of survival from fission. The excited nucleus then decays to the ground state via the emission of one or two neutrons only.209Bi(70Zn,xn)279-xUut (x=1)
The synthesis of ununtrium was first attempted in 1998 by the team at GSI using the above cold fusion reaction. In two separate runs, they were unable to detect any atoms and calculated a cross section limit of 900 fb
Barn (unit)
A barn is a unit of area. Originally used in nuclear physics for expressing the cross sectional area of nuclei and nuclear reactions, today it is used in all fields of high energy physics to express the cross sections of any scattering process, and is best understood as a measure of the...
.
They repeated the experiment in 2003 and lowered the limit further to 400 fb.
In late 2003, the emerging team at RIKEN
RIKEN
is a large natural sciences research institute in Japan. Founded in 1917, it now has approximately 3000 scientists on seven campuses across Japan, the main one in Wako, just outside Tokyo...
using their efficient apparatus GARIS attempted the reaction and reached a limit of 140 fb. In December 2003 – August 2004, they resorted to 'brute force' and performed an eight-month-long irradiation in which they increased the sensitivity to 51 fb. They were able to detect a single atom of 278Uut.
They repeated the reaction in several runs in 2005 and were able to synthesize a second atom. They calculated a record-low 31 fb for the cross section for the 2 atoms. The reaction was repeated again in 2006 with two long production runs but no further atoms were detected. This lowered the yield further to the current value of just 23 fb.
Hot fusion
This section deals with the synthesis of nuclei of ununtrium by so-called "hot" fusion reactions. These are processes which create compound nuclei at high excitation energy (~40–50 MeV, hence "hot"), leading to a reduced probability of survival from fission. The excited nucleus then decays to the ground state via the emission of 3–5 neutrons. Fusion reactions utilizing 48Ca nuclei usually produce compound nuclei with intermediate excitation energies (~30–35 MeV) and are sometimes referred to as "warm" fusion reactions. This leads, in part, to relatively high yields from these reactions.237Np(48Ca,xn)285-xUut (x=3)
In June 2006, the Dubna-Livermore team synthesised ununtrium directly in the "warm" fusion reaction between neptunium-237 and calcium-48 nuclei. Two atoms of 282Uut were detected with a cross section of 900 fb.
As a decay product
Ununtrium has also been detected in the decay of ununpentiumUnunpentium
Ununpentium is the temporary name of a synthetic superheavy element in the periodic table that has the temporary symbol Uup and has the atomic number 115....
and ununseptium
Ununseptium
Ununseptium is the temporary name of a superheavy artificial chemical element with temporary symbol Uus and atomic number 117. Six atoms were detected by a joint Russia–US collaboration at Dubna, Moscow Oblast, Russia, in 2009–10...
.
Chronology of isotope discovery
| Isotope | Year discovered | Discovery reaction |
|---|---|---|
| 278Uut | 2004 | 209Bi(70Zn,n) |
| 279Uut | unknown | |
| 280Uut | unknown | |
| 281Uut | unknown | |
| 282Uut | 2006 | 237Np(48Ca,3n) |
| 283Uut | 2003 | 243Am(48Ca,4n) |
| 284Uut | 2003 | 243Am(48Ca,3n) |
| 285Uut | 2009 | 249Bk(48Ca,4n) |
| 286Uut | 2009 | 249Bk(48Ca,3n) |
Cold fusion
The table below provides cross-sections and excitation energies for cold fusion reactions producing ununtrium isotopes directly. Data in bold represent maxima derived from excitation function measurements. + represents an observed exit channel.| Projectile | Target | CN | 1n | 2n | 3n |
|---|---|---|---|---|---|
| 70Zn | 209Bi | 279Uut | 23 fb |
Hot fusion
The table below provides cross-sections and excitation energies for hot fusion reactions producing ununtrium isotopes directly. Data in bold represents maxima derived from excitation function measurements. + represents an observed exit channel.| Projectile | Target | CN | 3n | 4n | 5n |
|---|---|---|---|---|---|
| 48Ca | 237Np | 285Uut | 0.9 pb Barn (unit) A barn is a unit of area. Originally used in nuclear physics for expressing the cross sectional area of nuclei and nuclear reactions, today it is used in all fields of high energy physics to express the cross sections of any scattering process, and is best understood as a measure of the... , 39.1 MeV |
Evaporation residue cross sections
The below table contains various targets-projectile combinations for which calculations have provided estimates for cross section yields from various neutron evaporation channels. The channel with the highest expected yield is given.DNS = Di-nuclear system; σ = cross section

