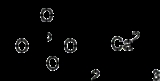
Tricalcium phosphate
Overview
Calcium
Calcium is the chemical element with the symbol Ca and atomic number 20. It has an atomic mass of 40.078 amu. Calcium is a soft gray alkaline earth metal, and is the fifth-most-abundant element by mass in the Earth's crust...
salt of phosphoric acid
Phosphoric acid
Phosphoric acid, also known as orthophosphoric acid or phosphoric acid, is a mineral acid having the chemical formula H3PO4. Orthophosphoric acid molecules can combine with themselves to form a variety of compounds which are also referred to as phosphoric acids, but in a more general way...
with the chemical formula
Chemical formula
A chemical formula or molecular formula is a way of expressing information about the atoms that constitute a particular chemical compound....
Ca3(PO4)2. It is also known as tribasic calcium phosphate or "bone ash" (calcium phosphate being one of the main combustion
Combustion
Combustion or burning is the sequence of exothermic chemical reactions between a fuel and an oxidant accompanied by the production of heat and conversion of chemical species. The release of heat can result in the production of light in the form of either glowing or a flame...
products of bone
Bone
Bones are rigid organs that constitute part of the endoskeleton of vertebrates. They support, and protect the various organs of the body, produce red and white blood cells and store minerals. Bone tissue is a type of dense connective tissue...
).
It has an alpha and a beta crystal form, the alpha state being formed at high temperatures. As rock, it is found in Whitlockite
Whitlockite
Whitlockite is a mineral, an unusual form of calcium phosphate. Its formula is Ca96PO3OH. It is a relatively rare mineral but is found in granitic pegmatites, phosphate rock deposits, guano caves and in chondrite meteorites...
.
The name calcium phosphate
Calcium phosphate
Calcium phosphate is the name given to a family of minerals containing calcium ions together with orthophosphates , metaphosphates or pyrophosphates and occasionally hydrogen or hydroxide ions ....
refers to minerals containing calcium ions (Ca2+) together with orthophosphates (PO43-), metaphosphate
Metaphosphate
A metaphosphate ion is an oxyanion that has the empirical formula PO3−. The structure of a metaphosphate ion can be described as being made up of PO4 structural units in which each unit shares two corners with another unit...
s or pyrophosphate
Pyrophosphate
In chemistry, the anion, the salts, and the esters of pyrophosphoric acid are called pyrophosphates. Any salt or ester containing two phosphate groups is called a diphosphate. As a food additive, diphosphates are known as E450.- Chemistry :...
s (P2O74-) and occasionally hydrogen or hydroxide
Hydroxide
Hydroxide is a diatomic anion with chemical formula OH−. It consists of an oxygen and a hydrogen atom held together by a covalent bond, and carrying a negative electric charge. It is an important but usually minor constituent of water. It functions as a base, as a ligand, a nucleophile, and a...
ions.
Especially, the common mineral apatite
Apatite
Apatite is a group of phosphate minerals, usually referring to hydroxylapatite, fluorapatite, chlorapatite and bromapatite, named for high concentrations of OH−, F−, Cl− or Br− ions, respectively, in the crystal...
has formula Ca5(PO4)3X, where X is F
Fluoride
Fluoride is the anion F−, the reduced form of fluorine when as an ion and when bonded to another element. Both organofluorine compounds and inorganic fluorine containing compounds are called fluorides. Fluoride, like other halides, is a monovalent ion . Its compounds often have properties that are...
, Cl
Chloride
The chloride ion is formed when the element chlorine, a halogen, picks up one electron to form an anion Cl−. The salts of hydrochloric acid HCl contain chloride ions and can also be called chlorides. The chloride ion, and its salts such as sodium chloride, are very soluble in water...
, OH
Hydroxide
Hydroxide is a diatomic anion with chemical formula OH−. It consists of an oxygen and a hydrogen atom held together by a covalent bond, and carrying a negative electric charge. It is an important but usually minor constituent of water. It functions as a base, as a ligand, a nucleophile, and a...
, or a mixture; it is hydroxyapatite if the extra ion is mainly hydroxide.

